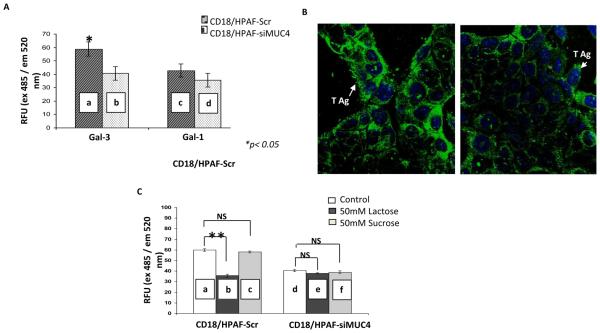
Figure 2. MUC4-galectin-3 interaction is glycosylation dependent and MUC4 possesses T antigen structures on its surface.
A, cell adhesion assay showed a significant difference between the binding of CD18/HPAF-SCR and CD18/HPAF-siMUC4 cells (a and b) to galectin-3 coated plates (*P<0.05). But, there was no difference in the case of galectin-1-coated plates (c and d). B, immunofluorescence analysis using T antigen specific Lectin PNA-Alexa Fluor 488 conjugates showed more intense T antigen staining on CD18/HPAF-Scr cells than MUC4 reduced CD18/HPAF-si-MUC4 cells. C, cell adhesion assay in the presence of lactose (a competitive inhibitor) and sucrose (a non-competitive inhibitor) showed involvement of carbohydrate structures in MUC4-gelectin-3 interaction. There is a significant decrease in the number of cells adhered to galectin-3-coated plates (**P=8.66E-11) only in the case of CD18/HPAF-Scr cells, in the presence of lactose (a and b). Micrographs shown are representative of several independent experiments.