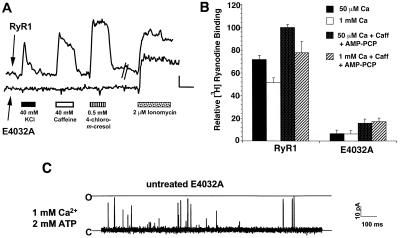
Figure 2.
E4032A channels are largely unresponsive to activation by RyR agonists. (A) 1B5 cells grown in collagen-coated 72-well microtiter plates (Terasaki format) were infected with either E4032A or wtRyR1 cDNA containing herpes simplex virions at 3 × 105 IU/ml. Cells were examined for calcium responses by using Fura-2 as described in Materials and Methods. The change in cytoplasmic calcium (as indicated by a change in F340/F380 ratio for Fura-2) in response to 40 mM KCl, 40 mM caffeine, 0.5 mM 4-chloro-m-cresol, or 2 μM ionomycin for either a wtRyR1-expressing myotube (upper trace) or E4032A-expressing myotube (lower trace) is indicated. (Bar = 0.1 340/380 ratio units vs. 50 sec.) (B) E4032A RyR1 shows largely reduced high-affinity [3H]ryanodine binding. wtRyR1 or E4032A RyR1-expressing 1B5 membrane preparations were incubated at 37°C for 3 h in a buffer containing 250 mM KCl, 15 mM NaCl, 20 mM Pipes (pH 7.4), and 10 nM [3H]ryanodine. Caffeine (20 mM) and 1 mM AMP-PCP and/or CaCl2 was added as indicated in the graph. The relative binding is calculated as the percentage of the binding of wtRyR1 in the presence of 50 μM CaCl2, 20 mM caffeine, and 1 mM AMP-PCP. The experiment has been repeated at least twice in duplicate. (C) Single channel measurements of isolated E4032A channels reconstituted in BLM were conducted as described in Materials and Methods. Isolated E4032A channels give rise to infrequent gating transitions from the closed to fully open state in the presence of cis 1 mM calcium and 2 mM ATP. The open probability of this channel was 0.0022.