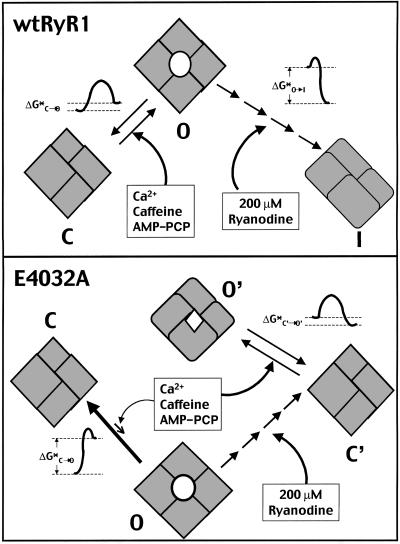
Figure 6.
Proposed model showing the interaction between ryanodine and either the wtRyR1 or the E4032A RyR mutant. With wtRyR, agents that increase channel Po are hypothesized to reduce the free energy (ΔG*C→O) associated with the transition from closed to open conformations. A high concentration of ryanodine promotes sequential binding to allosterically coupled sites which bring the channel into a persistently inhibited state with a large energy barrier for transition to the open state (ΔG*I→O). By contrast, E4032A exhibits a large energy barrier that is not affected by Ca2+, caffeine, and AMP-PCP, singly or in combination. A high concentration of ryanodine promotes sequential binding to allosterically coupled sites on E4032A, but the outcome is a dramatic decrease in free energy associated with channel gating between closed (C′) and open (O′) states in the ryanodine-modified E4032A RyR1.