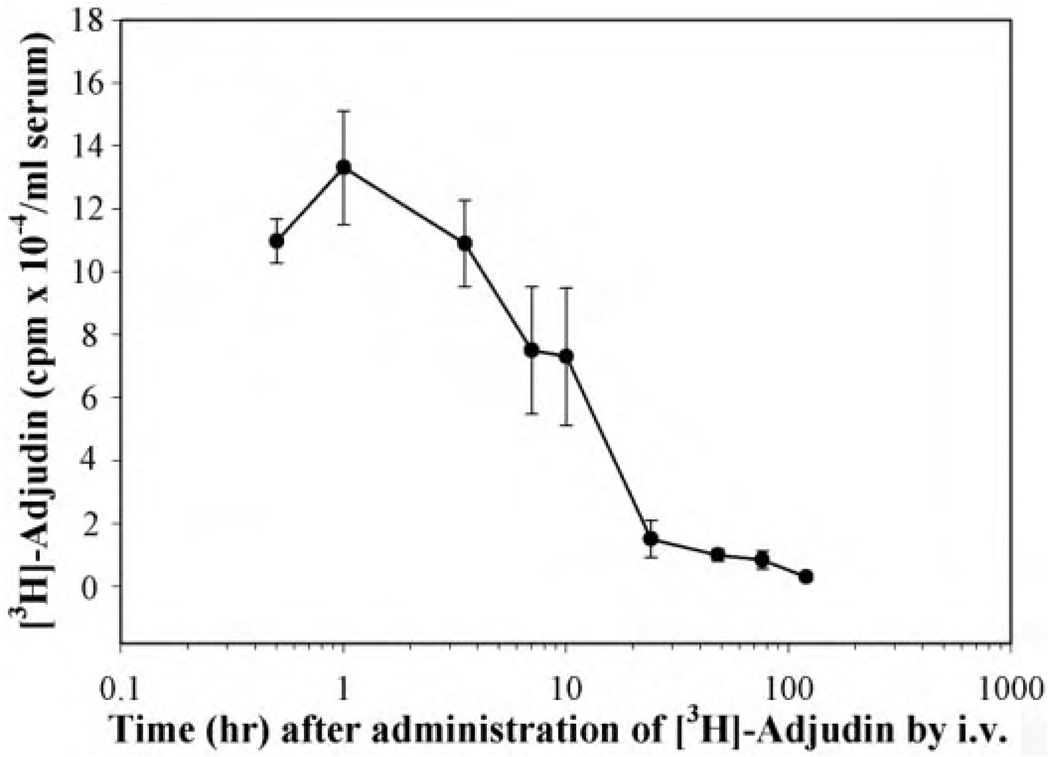
FIG. 10.
Estimation of the half-time of disappearance of [3H]adjudin in adult rats after i.v. administration. The half-time of disappearance of adjudin was estimated in adult rats (~300 g b.wt., n = 3 per time point) after i.v. administration of [3H]adjudin. In brief, [3H]adjudin, [indazole-5,7-3H(N)]-1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, was purchased (PerkinElmer Life and Analytical Sciences, Waltham, MA). The purity of [3H]adjudin was confirmed by 1) high-performance liquid chromatography using a Zorbax C18 reversed-phase column, which had an identical retention time when both 3H-labeled and unlabeled adjudin were injected onto the column simultaneously or separately with the eluents monitored by UV absorbance at 210 nm or spectrophotometry using a β-counter, 2) mass spectrometry, and 3) elemental analysis. The t1/2 of adjudin in the systemic circulation of adult rats was estimated by injecting ~3 × 106 cpm of [3H]adjudin in a sample volume of 50 µl of phosphate-buffered saline via the jugular vein. An aliquot of blood was collected from the tail vein from each rat in this experiment at 0.5, 1, 3.5, 7, 10, 24, 48, 76, and 120 h after administration of [3H]adjudin and was allowed to clot. Serum was obtained by centrifugation for radioactivity determination. The t1/2 was determined using nonlinear least-squares curve-fitting techniques to fit [3H]adjudin levels in blood as a function of time to a multiexponential function consisting of one to four terms of the following equation: Y(t) = ΣAie−Bit, where Y(t) is the response variable (in this case, the level of [3H]adjudin obtained in the blood sample). Data were fitted using a computer program based on code implementing the Marquardt algorithm to minimize χ2 (Bevington, 1969). Because data exhibited nonuniform variation, they were weighted as 1/σ2, where σ was determined from samples of three different animals at each time point. The number of exponentials fitted to experimental data was determined as the number that minimized χ2. Estimates were obtained for the parameters σAi and σBi, as well as their uncertainties Ai and Bi, which were derived from the diagonal terms in the error matrix generated during the fitting procedure. Note that the estimates for the parameter uncertainties did not take into account covariance terms and, as a result, tended to be underestimated. However, this analysis yielded the best estimate on the disappearance of [3H]adjudin from the systemic circulation after i.v. administration.