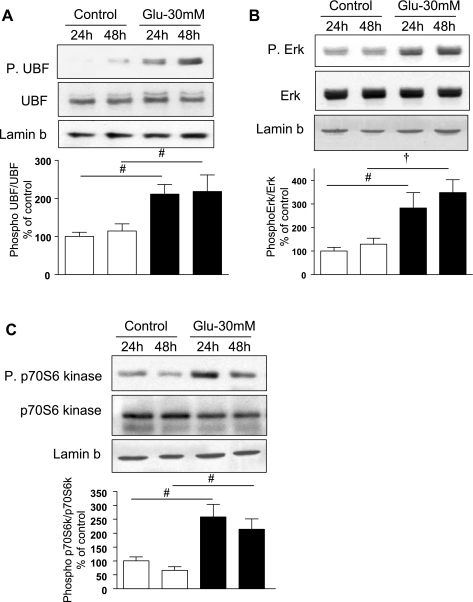
Fig. 2.
High glucose stimulates phosphorylation (P) of upstream binding factor 1 (UBF1), Erk1/2 MAP kinase, and p70S6 kinase in the nuclear compartment. Equal amounts of protein from nuclear extracts from cells treated with or without high glucose for 24 and 48 h were immunoblotted with antibodies that specifically detect phosphorylated forms of UBF1, Erk, and p70S6 kinase. Phosphorylation of UBF1 (A), Erk (B), and p70S6 kinase (C) was increased upon incubation with high glucose. Immunoblotting with antibodies against UBF, Erk, and p70S6 kinase is shown to evaluate changes in the respective proteins. Immunoblotting with antibody against lamin b, a nuclear marker, was performed to assess loading and establish purity of the nuclear preparation (top). Representative blots for each kinase from 3–5 experiments are shown, and composite data are given in histograms. #P < 0.05 and †P < 0.01 vs. control by ANOVA.