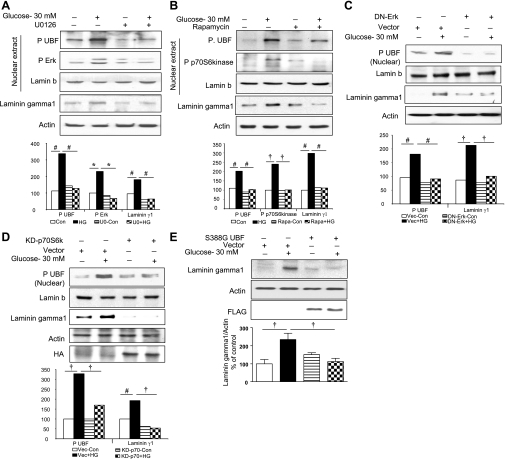
Fig. 3.
A–D: high-glucose (HG)-induced UBF1 phosphorylation and synthesis of laminin γ1 protein are dependent on Erk and p70S6 kinase phosphorylation. Serum-starved GEC were incubated with high glucose for 24 h with or without preincubation with 5 μM U-0126 (U0), an inhibitor of MEK, the upstream kinase of Erk (A), or 22 nM rapamycin (Rapa), an inhibitor of mammalian target of rapamycin complex 1 [mTORC1]-dependent phosphorylation of p70S6 kinase (B). C and D: GECs transfected with inactive dominant-negative (DN) mutant of Erk and kinase-dead p70S6 kinase mutant, respectively, followed by treatment with 30 mM glucose for 24 h. Equal amounts of nuclear extracts were resolved on SDS-PAGE and immunoblotted with phosphospecific antibodies for UBF1, Erk, and p70S6 kinase. Total cell lysates were probed for laminin γ1 content by immunoblotting. Lamin b and actin immunoblot analysis of the same samples was performed to assess loading in nuclear extracts and total lysates, respectively. Immunoblotting for hemagglutinin (HA) (D, top) was performed to assess transfection efficiency. Representative blots from 3 independent experiments are shown, and histograms represent means ± SE from 3 experiments. Statistically significant differences are indicated by #P < 0.05, †P < 0.01, and *P < 0.001 by ANOVA. E: laminin γ1 synthesis induced by high glucose requires UBF1 phosphorylation. GEC were transfected with 1 μg of a transcriptionally inactive UBF1 mutant (S388G)/well or an empty vector (Vec) without the construct as control and allowed to grow for 24 h. Cells were treated with or without 30 mM glucose for 24 h, and laminin γ1 protein was detected as described in Fig. 1B. Immunoblotting for actin (middle) and FLAG (bottom) was performed to assess loading and transfection efficiency, respectively. A representative blot from 4 experiments is shown along with quantitative data from all experiments (†P < 0.01 by ANOVA).