Abstract
Aging is associated with an increase in oxidative stress and blood pressure (BP). Renal dopamine D1 (D1R) and angiotensin II AT1 (AT1R) receptors maintain sodium homeostasis and BP. We hypothesized that age-associated increase in oxidative stress causes altered D1R and AT1R functions and high BP in aging. To test this, adult (3 mo) and old (21 mo) Fischer 344 × Brown Norway F1 rats were supplemented without/with antioxidant tempol followed by determining oxidative stress markers (urinary antioxidant capacity, proximal tubular NADPH-gp91phox, and plasma 8-isoprostane), D1R and AT1R functions, and BP. The D1R and AT1R functions were determined by measuring diuretic and natriuretic responses to D1R agonist (SKF-38393; 1 μg·kg−1·min−1 iv) and AT1R antagonist (candesartan; 10 μg/kg iv), respectively. We found that the total urinary antioxidant capacity was lower in old rats, which increased with tempol treatment. In addition, tempol decreased the elevated NADPH-gp91phox and 8-isoprostane levels in old rats. Systolic, diastolic, and mean arterial BPs were higher in old rats and were reduced by tempol. Although SKF-38393 produced diuresis in both adult and old rats, urinary sodium excretion (UNaV) increased only in adult rats. While candesartan increased diuresis and UNaV in adult and old rats, the magnitude of response was greater in old rats. Tempol treatment in old rats reduced candesartan-induced increase in diuresis and UNaV. Our results demonstrate that diminished renal D1R and exaggerated AT1R functions are associated with high BP in old rats. Furthermore, oxidative stress may cause altered renal D1R and AT1R functions and high BP in old rats.
Keywords: aging, sodium, dopamine, angiotensin
hypertension is a major risk factor for cardiovascular morbidity and mortality (12, 34, 41, 42, 45). There are genetic and nongenetic factors implicated in the development of hypertension (33). Defective sodium handling by the kidney has also been linked in the pathogenesis of hypertension, suggesting a relationship between sodium excretion and blood pressure (BP) (19, 24, 26, 32).
Kidney dopamine and angiotensin II (ANG II) are two key counterregulatory endogenous compounds that play pivotal roles in the maintenance of sodium homeostasis and BP (23). Dopamine exerts its natriuretic action primarily via D1-like (comprised of D1 and D5 subtypes) receptors and contributes to a majority of the sodium excretion during increased sodium intake (11, 27, 28). Impairments in natriuretic and diuretic responses to D1-like receptor agonists are linked to hypertension in humans and rodents (1, 13, 14, 17, 22, 29, 48). In contrast, ANG II mediates its antinatriuretic effects via ANG II type 1 (AT1) receptors, whereas activation of ANG II type 2 (AT2) receptors produces natriuresis (30, 31, 35). Within the kidney, 95% of the receptors are of the AT1 subtype, altered functioning of which has also been linked to various forms of hypertension (9, 40). Besides their counterregulatory actions on sodium transporters, recent studies suggest a direct antagonism between the dopamine and renin-angiotensin system in regulating sodium balance (23).
There are studies demonstrating that BP increases with advancing age (36, 38, 43). In the elderly, hypertension is implicated in myocardial infarction, stroke, and heart and kidney dysfunctions (38). A host of structural and functional changes in the kidney accompanies the aging process, which are often associated with an inability of the kidney to regulate sodium balance (20, 37). Aging is also associated with an increase in oxidative stress (2) and impairments in renal dopamine D1R function (10, 47). The role of oxidative stress in causing renal dopamine D1R dysfunction in old rats has been previously established (4, 7, 21). However, role of oxidative stress in altering renal AT1R function in aging has not been investigated. Since both renal dopamine (via D1-like receptors) and ANG II (via AT1 receptors) are regulators of sodium homeostasis and their impaired functions are linked to hypertension, we hypothesized that hypertension in aging is associated with altered renal D1 and AT1 receptor functions and age-associated oxidative stress plays causative role in this phenomenon.
Therefore, in the present study, we investigated the effects of age-associated oxidative stress on BP, natriuretic and diuretic responses to D1-like receptor agonist SKF-38393 and AT1 receptor antagonist candesartan. The role of age-associated oxidative stress was determined by supplementing adult (3 mo) and old (21 mo) Fischer 344 × Brown Norway F1 (FBN) rats with antioxidant tempol. These rats are widely used as rat aging model as they closely resemble age-related changes as in humans (39).
METHODS
Animals
Adult (3 mo) and old (21 mo) male FBN hybrid rats raised by Harlan Sprague-Dawley (Indianapolis, IN) were purchased from the National Institute on Aging (Bethesda, MD). These rats were housed in plastic cages in the University of Houston animal care facility and were used as per the National Institutes of Health guidelines and approved protocols by the Institutional Animal Care and Use Committee. Animals had free access to the standard rodent chow and drinking water.
Tempol Supplementation
Adult and old rats were provided with tempol (Sigma; 1 mM in drinking water) for a duration of 3–4 wk. Control adult and old rats received drinking water. Treated adult and old rats had free access to tempol-supplemented water, which was changed twice a day to minimize its oxidation.
Animal Surgery
The animals were anesthetized with inactin (150 μg/kg body wt ip) and tracheotomy was performed to facilitate breathing. To measure BP and collection of blood samples, left carotid artery was catheterized with PE-50 tubing connected to a pressure transducer. BP was continuously recorded on a Grass Polygraph (model 7D; Grass Instrument, Quincy, MA) throughout the experiment. The left jugular vein was also catheterized with PE-50 tubing for infusion of drug/saline. A midline abdominal incision was made, bladder urine was collected with a syringe, and left ureter was catheterized to collect urine through PE-10 tubing during saline/drug infusion. The rats were continuously infused with normal saline (1% body wt ml/h) for the duration of the surgery to prevent dehydration and maintain a stable urinary output. Bladder urine was used to measure total antioxidant capacity.
Renal Function Studies
SKF-38393.
The protocol consisted of stabilizing the animals for 45 min after the surgery followed by three consecutive 30-min urine collection periods viz. C1, C2 (averaged as C), and D. Collections C1 and C2 (basal) correspond to periods where only saline was infused, whereas D corresponds to the period where SKF-38393 (1 μg·kg−1·min−1 iv) in saline was infused. Urine samples were collected throughout the 30-min period, whereas blood samples (250 μl) were collected at the end of each C2 and D collection periods. Equal volume of saline was infused to replace the blood volume.
Candesartan.
Similar to the above protocol, animals were stabilized for 45 min followed by six consecutive 30-min urine collection periods viz. C1, C2 (averaged as C), D1, D2, D3, and D4. During C1 and C2, only saline was infused to determine the basal parameters. Candesartan was administered as a bolus dose (10 μg/kg body wt iv) and D1-D4 represent collections following drug treatment. Urine samples were collected throughout the 30-min period and blood samples at C2, D2, and D4 periods (250 μl; replaced with equal volume of saline) were collected as above.
Blood plasma was obtained by centrifuging blood samples at 1,500 g for 15 min at 4°C. Urine and plasma samples were stored at −80°C for further analyses.
Urine and Plasma Analysis
Sodium concentration in the urine and plasma was measured using an AAnalyst 400 atomic absorption spectrometer (Perkin Elmer, Waltham, MA). Creatinine levels in the plasma and urine were measured using a commercially available assay kit (catalog no. K625–100; BioVision, Mountain View, CA).
Evaluation of Renal Function
Urine volume was measured gravimetrically and urinary flow was assessed (μl/min). Urinary sodium excretion (UNaV; μmol/min) was calculated based on urine flow and urinary sodium concentration [urine flow (μl/min)*urinary sodium concentration (μmol/μl)]. The glomerular filtration rate (ml/min) was determined based on the clearance of creatinine {[urine flow (ml/min)*urine creatinine (mg/dl)]/plasma creatinine (mg/dl)}.
Preparation of Renal Proximal Tubules
An in situ enzyme digestion procedure was used to prepare renal proximal tubules. Briefly, a midline abdominal incision was made, aorta was catheterized with PE-50 tubing, and kidneys were perfused with collagenase and hyaluronidase. The kidneys were removed and kept in ice-cold oxygenated Krebs buffer containing (in mM) 1.5 CaCl2, 110 NaCl, 5.4 KCl, 1 KH2PO4, 1 MgSO4, 25 NaHCO3, 25 d-glucose, and 2 HEPES (pH 7.4). Coronal sections of the kidneys were obtained, and superficial cortical tissue slices (rich in proximal tubules) were dissected out with a razor blade. The cortical slices were kept in fresh Krebs buffer. Enrichment of proximal tubules was carried out using 25% Ficoll in Krebs buffer. The band at Ficoll interface was collected and washed in Krebs buffer by centrifugation at 250 g for 5 min. Tubular cells' viability was performed using the Trypan blue exclusion test.
Measurement of NADPH-gp91phox and β-Actin
NADPH-gp91phox and β-actin (as loading control) were measured in the renal proximal tubular homogenate by standard Western blotting using specific anti-gp91phox antibody (BD Biosciences, San Jose, CA) and anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA), respectively, followed by horseradish peroxidase-conjugated goat-anti-mouse secondary antibody (Santa Cruz Biotechnology).
Measurement of 8-Isoprostane
8-Isoprostane was measured in plasma using a commercially available EIA-based kit (Cayman Chemical, Ann Arbor, MI). Briefly, this assay is based on the competition between 8-isoprostane and an 8-isoprostane-acetylcholinesterase (AChE) conjugate (8-isoprostane tracer) for a limited number of 8-isoprostane-specific rabbit antiserum binding sites. The amount of 8-isoprostane tracer bound (which is inversely proportional to the concentration of 8-isoprostane in the sample) to the rabbit antiserum is determined by adding Ellman's reagent (which contains the substrate for AChE) and the intensity of the developed yellow color is measured spectrophotometrically at 412 nm.
Total Antioxidant Capacity
The total antioxidant capacity in bladder urine was determined using a commercially available kit according to the manufacturer's protocol (catalog no. 709001; Cayman Chemical). Briefly, the assay relies on the ability of antioxidants in the sample to inhibit the oxidation of 2,2′-azino-di-[3-ethylbenzthiazoline sulphonate] (ABTS®) to ABTS®.+ by metmyoglobin. The capacity of the antioxidants in the sample to prevent ABTS® oxidation is compared with that of Trolox, a water-soluble tocopherol analog, and is quantified as millimolar Trolox equivalents.
Statistics
Data are presented as means ± SE. Repeated-measures ANOVA followed by Dunnett post hoc test was used to compare variations (from control) within the group. One-way ANOVA followed by Newman-Keuls post hoc test was used to compare variations among the groups. Also, paired Student's t-test was used wherever appropriate. Statistical analysis was carried out using a software program (GraphPad Prism ver. 5; GraphPad Software, San Diego, CA). The minimum level of significance was considered at P < 0.05.
RESULTS
Effect of Aging on Body Weight and Food and Water Intake
The body (adult vs. old rats: 259.1 ± 9.65 vs. 578.5 ± 14.65 g) and kidney (adult vs. old rats: 0.915 ± 0.04 vs. 1.55 ± 0.03 g) weights were significantly higher in old compared with adult rats. However, food (adult vs. old rats: 21.9 ± 0.9 vs. 21.81 ± 0.95 g·rat−1·day−1) and water (adult vs. old rats: 29.44 ± 1.54 vs. 25.12 ± 1.74 ml·rat−1·day−1) intakes were not different between these two groups.
Effect of Aging on Antioxidant Capacity
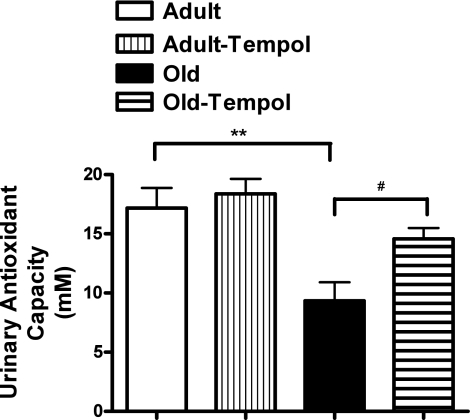
As shown in Fig. 1, the urinary antioxidant capacity was lower in old than in adult rats. Tempol treatment increased the urinary antioxidant capacity in old rats. However, tempol treatment did not change the antioxidant capacity in adult rats.
Fig. 1.
Antioxidant tempol increases age-related decline in antioxidant capacity in old rats. Total antioxidant capacity in the urine was measured using a kit-based assay (details in methods). Results are means ± SE (n = 6 rats in each group). **Significantly different from adult rats at P < 0.01. #Significantly different from old rats at P < 0.05 (1-way ANOVA followed by Newman-Keuls post hoc test).
Effect of Aging on Renal Proximal Tubular NADPH-gp91phox
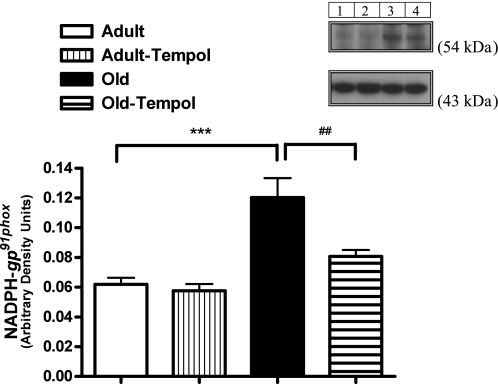
As shown in Fig. 2, the levels of NADPH-gp91phox (normalized to β-actin) were higher in old compared with adult rats. Tempol treatment reduced the levels of NADPH-gp91phox in old but not in adult rats.
Fig. 2.
Antioxidant tempol decreases age-related increase in NADPH-gp91phox in the proximal tubular homogenate in old rats. NADPH-gp91phox and β-actin were measured by Western blotting (details in methods). Results are means ± SE (n = 4–5 rats in each group). Top: representative blots of NADPH-gp91phox and β-actin (loading control). Lane 1: adult control; lane 2: adult tempol; lane 3: old control; lane 4: old tempol. Bottom: bars are ratios of the densities between NADPH-gp91phox and β-actin protein bands. ***Significantly different from adult rats at P < 0.001. ##Significantly different from old rats at P < 0.01 (1-way ANOVA followed by Newman-Keuls post hoc test).
Effect of Aging on Plasma 8-Isoprostane
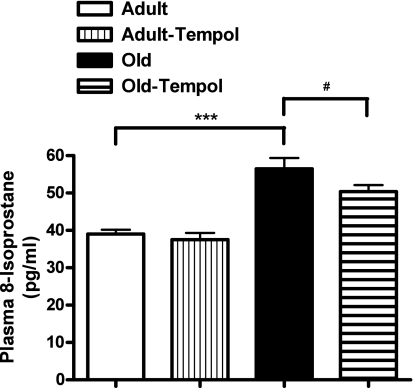
Plasma 8-isoprostane was significantly higher in old rats compared with adult rats (Fig. 3). Treatment with tempol reduced the levels of plasma 8-isoprostane in old rats. However, tempol treatment did not change the plasma 8-isoprostane levels in adult rats.
Fig. 3.
Antioxidant tempol decreases age-related increase in plasma 8-isoprostane levels in old rats. 8-Isoprostane in plasma was measured using an EIA-based kit assay (details in methods). Results are means ± SE (n = 6–8 rats in each group). ***Significantly different from adult rats at P < 0.001. #Significantly different from old rats at P < 0.05 (1-way ANOVA followed by Newman-Keuls post hoc test).
Effect of Aging on Dopamine D1 Receptor Function
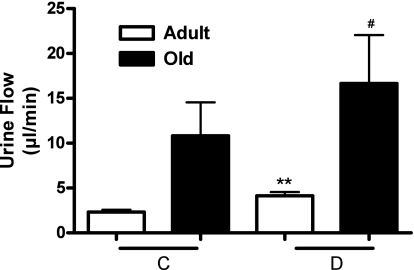
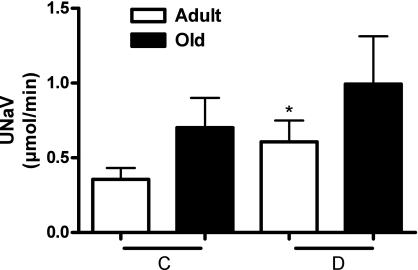
As shown in Fig. 4, D1-like receptor agonist SKF-38393 increased urine flow in both adult and old rats. However, UNaV in response to SKF-38393 increased significantly only in adult rats (Fig. 5).
Fig. 4.
Renal dopamine D1 receptor activation produces diuresis (urine flow) in adult and old rats. Urine flow in response to D1 receptor agonist SKF-38393 (1 μg·kg−1·min−1 iv) was measured in anesthetized rats (details in methods). C represents average of 2 30-min control periods where saline only was infused. D represents 30-min drug infusion period where SKF-38393 in saline was infused. Results are means ± SE (n = 6 adult and 8 old rats). **Significantly different from C in adult rats at P < 0.01. #Significantly different from C in old rats at P < 0.05. Statistical significance was achieved using paired t-test.
Fig. 5.
Renal dopamine D1 receptor activation produces natriuresis (UNaV) in adult but not in old rats. UNaV in response to D1 receptor agonist SKF-38393 (1 μg·kg−1·min−1 iv) was determined in anesthetized rats (details in methods). C and D as defined in Fig. 4. Results are means ± SE (n = 6 adult and 10 old rats). *Significantly different from C in adult rats at P < 0.05 (paired t-test).
Effect of Aging on AT1 Receptor Function
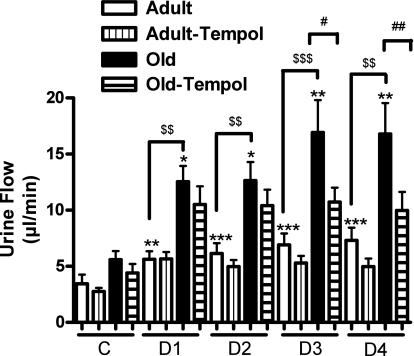
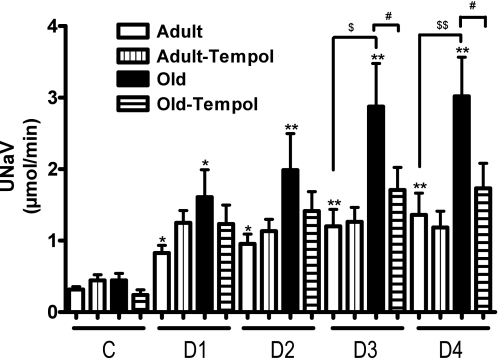
There was a significantly greater increase in urine flow in response to the AT1 receptor antagonist candesartan in old rats (Fig. 6). Similarly, there was an exaggerated response to candesartan on UNaV in old than in adult rats (Fig. 7). While tempol treatment had no effect in adult rats, the exaggerated diuretic (Fig. 6) and natriuretic (Fig. 7) responses to candesartan were reduced with tempol treatment in old rats.
Fig. 6.
Antioxidant tempol reduces exaggerated angiotensin II AT1 receptor-mediated diuresis (urine flow) in old rats. Urine flow in response to AT1 receptor antagonist candesartan (10 μg/kg iv bolus) was determined in anesthetized rats (details in methods). C as defined in Fig. 4. D1-D4 represent drug infusion periods (each 30 min) after candesartan was administered as a bolus dose. Results are means ± SE (n = 5–7 rats). Significantly different from C (basal) using repeated-measures ANOVA followed by Dunnett post hoc test (***P < 0.001, **P < 0.01, and *P < 0.05). Significantly different from adult rats using 1-way ANOVA followed by Newman-Keuls post hoc test ($$$P < 0.001 and $$P < 0.01). Significantly different from old rats using 1-way ANOVA followed by Newman-Keuls post hoc test (##P < 0.01 and #P < 0.05).
Fig. 7.
Antioxidant tempol reduces exaggerated angiotensin II AT1 receptor-mediated UNaV in old rats. UNaV in response to AT1 receptor antagonist candesartan (10 μg/kg iv bolus) was determined in anesthetized rats. C and D1-D4 as defined in Figs. 4 and 6. Results are means ± SE (n = 5–7 rats). Significantly different from C (basal) using repeated-measures ANOVA followed by Dunnett post hoc test (**P < 0.01 and *P < 0.05). Significantly different from adult using 1-way ANOVA followed by Newman-Keuls post hoc test ($$P < 0.01 and $P < 0.05). Significantly different from old using 1-way ANOVA followed by Newman-Keuls post hoc test (#P < 0.05).
Effect of Aging on BP and Heart Rate
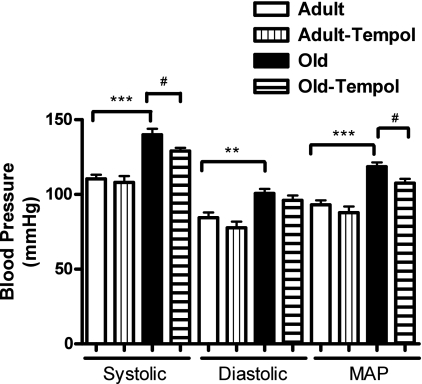
Systolic, diastolic, and mean arterial BPs were higher in old than in adult rats. Treatment with tempol reduced BP in old but not in adult rats (Fig. 8). However, heart rate (adult vs. old rats: 306.2 ± 15.57 vs. 330.3 ± 6.9 beats/min) was not different between adult and old rats.
Fig. 8.
Antioxidant tempol reduces age-related increase in blood pressure in old rats. Blood pressure (systolic, diastolic, and mean arterial) was measured in anesthetized rats (details in methods). Results are means ± SE (n = 6–10 rats). Significantly different from adult rats using 1-way ANOVA followed by Newman-Keuls post hoc test (***P < 0.001 and **P < 0.01). Significantly different from old rats using 1-way ANOVA followed by Newman-Keuls post hoc test (#P < 0.05).
DISCUSSION
Aging is frequently associated with high BP and its accompanied cardiovascular complications like myocardial ischemia, stroke, and heart failure (38). Since sodium retention is a hallmark for essential hypertension, a growing number of studies have focused on abnormal renal sodium handling in the pathogenesis of hypertension (19, 24, 26, 32). In the kidneys, dopamine and ANG II are two important regulators of sodium homeostasis and BP and serve counterregulatory functions in maintaining sodium balance (23). Our study clearly demonstrates an age-associated increase in BP and reduced D1 and exaggerated AT1 receptor function in FBN rats, an animal model widely used in aging research.
Aging is also associated with an increased state of oxidative stress (2). Our previous studies in old normotensive Fischer 344 rats (another rat model of aging) also showed increased oxidative stress with age (5, 8). This probably explains one of the most enduring theories of aging that implicates oxidative stress as a mediator of alterations in cellular function. There is emerging evidence that oxidative stress contributes to an increase in BP in various animal models of hypertension (9, 18, 44). However, most of this research highlighted the role of oxidative stress on vasculature especially in context to the development of atherosclerotic processes (25). In the kidney, which plays an integral part in maintenance of sodium homeostasis and regulation of BP, role of oxidative stress in the development of high BP as it relates to aging has not been clearly established. We previously explained the role of oxidative stress in causing impaired dopamine D1 receptor function in Fischer 344 rats (6, 8, 21). However, we did not study AT1 receptor function in this strain and additionally, these rats do not develop high BP with age. Therefore, in this study, we used FBN rats (rat aging model that exhibits hypertension) to determine the role of oxidative stress in contributing to age-related impairment in dopamine D1 and AT1 receptor functions and hypertension.
In this study, we found similar diuretic response to SKF-38393 (D1-like receptor agonist) in both adult and old rats. However, natriuretic response to SKF-38393 was reduced in old rats underscoring an age-related decline in renal dopamine D1 receptor function in these rats, an observation similar to that reported for Fischer 344 rats (21). In contrast, diuretic and natriuretic responses to candesartan (AT1 receptor antagonist) were much higher in old compared with adult rats. These changes in sodium excretion in response to candesartan suggest an exaggerated renal AT1 receptor function in old FBN rats. It should be noted that administration of either SKF-38393 or candesartan did not change hemodynamic parameters such as BP and glomerular filtration rate (data not shown), suggesting tubular-mediated effect of these drugs on sodium excretion. Taken together, these studies suggest that diminished D1 and exaggerated AT1 receptor function in the kidneys probably contributes to an increase in BP observed in old FBN rats.
To examine the role of oxidative stress, we treated these rats with antioxidant tempol and determined BP and natriuretic and diuretic responses to AT1 receptor antagonist candesartan. Tempol is a known antioxidant with superoxide dismutase mimetic properties and has been used previously to reduce oxidative stress and high BP in spontaneously hypertensive rats (46). We found an age-related increase in oxidative stress, determined by measuring three different oxidative stress markers in FBN rats. The levels of antioxidant capacity in urine were lower, whereas those of superoxide producing NADPH-gp91phox enzyme and 8-isoprostane in proximal tubules and plasma, respectively, were higher in old FBN rats. Tempol treatment in old rats increased the urinary antioxidant capacity and decreased the proximal tubular NADPH-gp91phox enzyme and plasma 8-isoprostane levels.
Furthermore, tempol administration reduced BP and normalized the exaggerated diuretic and natriuretic response to candesartan in old rats. This finding clearly establishes a causative role for oxidative stress in the development of age-dependent exaggerated renal AT1 receptor function and high BP. Also, natriuretic response to D1 receptor activation was diminished in old rats. In the present study, we did not measure natriuretic response to D1 receptor activation in tempol-supplemented adult and old rats. This was decided based on our previous finding in another rat aging model, Fischer 344 rats, demonstrating that tempol while reducing oxidative stress restores age-related decline in D1 receptor function (21). Therefore, it is likely that high BP phenotype in old FBN rats is associated with alteration in both renal D1 and AT1 receptor functions, most likely mediated via increased oxidative stress in these animals.
The mechanism of diminished renal D1 receptor function in old FBN rats is not known. Earlier we reported in Fischer 344 rats that age-associated oxidative stress via protein kinase C and G protein-coupled receptor kinase-2 pathway phosphorylates D1 receptors causing their uncoupling from G proteins and loss-of-functional responsiveness of the receptor (21). Therefore, it is likely that a similar mechanism of D1 receptor dysfunction in old FBN rats may exist, which needs to be determined.
With regard to exaggerated renal AT1 receptor function in old FBN rats, it is likely that age-associated oxidative stress increases the transcription and function of renal AT1 receptors. This notion is based on our earlier report demonstrating that age-related increase in oxidative stress in renal proximal tubules of old FBN rats is associated with higher NF-κB activity, increased AT1 receptor mRNA levels, and higher AT1 receptor function measured as the ability of ANG II to stimulate Na-K-ATPase activity (15). NF-κB is a redox-sensitive transcription factor (3) and AT1 receptor gene promoter is reported to have binding motif for NF-κB (16). Therefore, it is likely that age-associated oxidative stress mediates increase in AT1 receptor transcription via NF-κB and may represent the mechanism of exaggerated AT1 receptor function in old FBN rats. This is perhaps true as we found in the present study that reducing age-associated oxidative stress with antioxidant tempol reduces exaggerated AT1 receptor function in old FBN rats (Figs. 6 and 7). However, NF-κB activity and AT1 receptor mRNA levels still need to be determined in tempol-treated old FBN rats, which will be undertaken in future studies.
In summary, to our knowledge for the first time, we show causative role of age-associated oxidative stress in the development of high BP in FBN rats. The mechanism for this increase in BP may involve age-associated oxidative stress-mediated alterations in both renal dopamine D1 and AT1 receptor functions and subsequent tubular sodium handling contributing to high BP in old rats.
GRANTS
The study was financially supported by National Institutes of Health/National Institute on Aging Grants AG-25056 and AG-29904.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, Sibley DR, Westphal HJ, Jose PA. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J Clin Invest 97: 2283–2288, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90: 7915–7922, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asano S, Rice KM, Kakarla S, Katta A, Desai DH, Walker EM, Wehner P, Blough ER. Aging influences multiple indices of oxidative stress in the heart of the Fischer 344/NNia × Brown Norway/BiNia rat. Redox Rep 12: 167–180, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Asghar M, Banday AA, Fardoun RZ, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G protein-coupled receptor kinase 2. Free Radic Biol Med 40: 13–20, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Asghar M, Chillar A, Lokhandwala MF. Renal proximal tubules from old Fischer 344 rats grow into epithelial cells in cultures and exhibit increased oxidative stress and reduced D1 receptor function. Am J Physiol Cell Physiol 295: C1326–C1331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol 293: F914–F919, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Asghar M, Hussain T, Lokhandwala MF. Higher basal serine phosphorylation of D1A receptors in proximal tubules of old Fischer 344 rats. Am J Physiol Renal Physiol 283: F350–F355, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Asghar M, Lokhandwala MF. Antioxidant supplementation normalizes elevated protein kinase C activity in the proximal tubules of old rats. Exp Biol Med (Maywood) 229: 270–275, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Banday AA, Lokhandwala MF. Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol 295: F698–F706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beheray S, Kansra V, Hussain T, Lokhandwala MF. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int 58: 712–720, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38: 297–302, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med 76: 4–12, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Chen C, Beach RE, Lokhandwala MF. Dopamine fails to inhibit renal tubular sodium pump in hypertensive rats. Hypertension 21: 364–372, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Chen CJ, Lokhandwala MF. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin Exp Hypertens A 14: 615–628, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Chugh G, Lokhandwala M, Asghar M. High blood pressure in aged Fischer 344 × Brown Norway F1 rats is associated with increased oxidative stress, NFkB activation and exaggerated renal ATIR response to ANG II (Abstract). FASEB J 23: 1016.8, 2009 [Google Scholar]
- 16. Cowling RT, Zhang X, Reese VC, Iwata M, Gurantz D, Dillmann WH, Greenberg BH. Effects of cytokine treatment on angiotensin II type 1A receptor transcription and splicing in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol 289: H1176–H1183, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Damasceno A, Santos A, Serrao P, Caupers P, Soares-da-Silva P, Polonia J. Deficiency of renal dopaminergic-dependent natriuretic response to acute sodium load in black salt-sensitive subjects in contrast to salt-resistant subjects. J Hypertens 17: 1995–2001, 1999 [DOI] [PubMed] [Google Scholar]
- 18. De Champlain J, Wu R, Girouard H, Karas M, EL Midaoui A, Laplante MA, Wu L. Oxidative stress in hypertension. Clin Exp Hypertens 26: 593–601, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ 312: 1249–1253, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Epstein M. Aging and the kidney. J Am Soc Nephrol 7: 1106–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Fardoun RZ, Asghar M, Lokhandwala M. Role of oxidative stress in defective renal dopamine D1 receptor G protein coupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol 291: F945–F951, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Felder RA, Seikaly MG, Cody P, Eisner GM, Jose PA. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension 15: 560–569, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Gildea JJ. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr Opin Nephrol Hypertens 18: 28–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens 10: 49S–55S, 1997 [PubMed] [Google Scholar]
- 25. Hirooka Y, Sagara Y, Kishi T, Sunagawa K. Oxidative stress and central cardiovascular regulation. Pathogenesis of hypertension and therapeutic aspects. Circ J 74: 827–835, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Hollenberg NK, Moore T, Shoback D, Redgrave J, Rabinowe S, Williams GH. Abnormal renal sodium handling in essential hypertension. Relation to failure of renal and adrenal modulation of responses to angiotensin II. Am J Med 81: 412–418, 1986 [DOI] [PubMed] [Google Scholar]
- 27. Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 228: 134–142, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Jose PA, Eisner GM, Felder RA. Renal dopamine and sodium homeostasis. Curr Hypertens Rep 2: 174–183, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther 80: 149–182, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12: 70–88, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kuller LH. Salt and blood pressure: population and individual perspectives. Am J Hypertens 10: 29S–36S, 1997 [PubMed] [Google Scholar]
- 33. Kurokawa K, Okuda T. Genetic and nongenetic basis of essential hypertension: maladaptation of human civilization to high salt intake. Hypertens Res 21: 67–71, 1998 [DOI] [PubMed] [Google Scholar]
- 34. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335: 765–774, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Percy CJ, Power D, Gobe GC. Renal ageing: changes in the cellular mechanism of energy metabolism and oxidant handling. Nephrology (Carlton) 13: 147–152, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Pestana M. Hypertension in the elderly. Int Urol Nephrol 33: 563–569, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Rice KM, Wu M, Blough ER. Aortic aging in the Fischer 344/NNiaHSd × Brown Norway/BiNia rat. J Pharm Sci 108: 393–398, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+-K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens 28: 29–40, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Thom TJ. International mortality from heart disease: rates and trends. Int J Epidemiol 18: S20–S28, 1989 [PubMed] [Google Scholar]
- 42. Uemura K, Pisa Z. Trends in cardiovascular disease mortality in industrialized countries since 1950. World Health Stat Q 41: 155–178, 1988 [PubMed] [Google Scholar]
- 43. Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA 287: 1003–1010, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Vaziri ND. Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis 2: 1–10, 2008 [PubMed] [Google Scholar]
- 45. Whelton PK. Epidemiology of hypertension. Lancet 344: 101–106, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60: 418–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zemel MB, Sowers JR. Salt sensitivity and systemic hypertension in the elderly. Am J Cardiol 61: 7H–12H, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Zeng C, Eisner GM, Felder RA, Jose PA. Dopamine receptor and hypertension. Curr Med Chem Cardiovasc Hematol Agents 3: 69–77, 2005 [DOI] [PubMed] [Google Scholar]