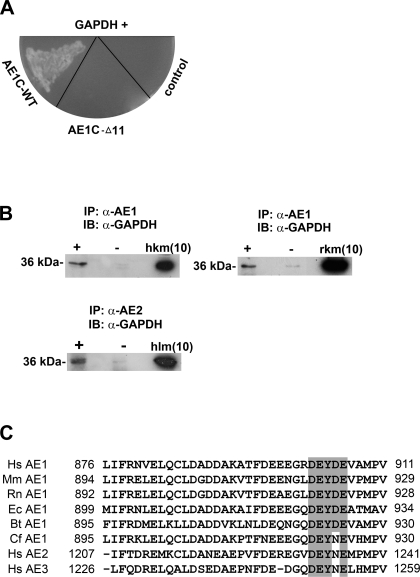
Fig. 1.
C-terminal domains of chloride-bicarbonate exchangers (AE1 and AE2) interact with GAPDH. A: following yeast two-hybrid screening of a human kidney cDNA library using either AE1C-wild-type (WT) or AE1C-Δ11 as bait, positive clones were verified by mating analysis on -Ade/-His/-Leu/-Trp/X-β-Gal drop-out plates. Two clones (one shown) corresponding to GAPDH displayed mating with AE1C-WT-containing cells, but not with cells harboring AE1C-Δ11. B: solubilized membrane protein fractions of fresh frozen human (top left) or rat (top right) kidney were immunoprecipitated using α-AE1 antibody (+ lanes). Detection of GAPDH (∼36 kDa) by α-GAPDH antibody indicates coimmunoprecipitation in both species. Similarly, samples prepared from human liver membrane were immunoprecipitated using specific α-AE2 antibody followed by detection of GAPDH (bottom). − Lanes indicate omission of α-AE1 or α-AE2 antibody. IP, immunoprecipitation; IB, immunoblot; hkm/rkm, human/rat kidney membrane; hlm, human liver membrane protein (inputs). Numbers in brackets indicate amounts (μg) of samples loaded. C: comparative sequence analysis of the C termini of AEs 1–3 from human (Hs), mouse (Mm), rat (Rn), horse (Ec), cow (Bt), and dog (Cf) reveals high homology, especially around Y904 of AE1. Residues at the start and end of this domain are numbered. The motif containing key residues for AE1/GAPDH binding (see Fig. 3) are shaded, and conserved amino acids within it are highlighted.