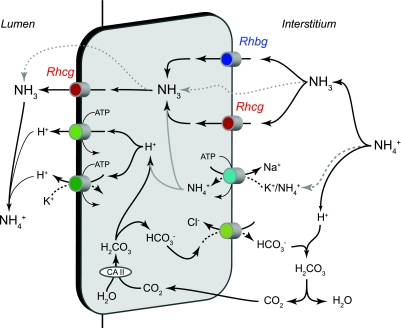
Fig. 6.
Model of collecting duct ammonia secretion. In the interstitium, NH4+ is in equilibrium with NH3 and H+. NH3 is transported across the basolateral membrane through both Rhesus glycoproteins Rhbg and Rhcg. In the IMCD, basolateral Na+-K+-ATPase is a major mechanism of basolateral NH4+ uptake, followed by dissociation of NH4+ to NH3 and H+ (grey lines). Intracellular NH3 is secreted across the apical membrane by apical Rhcg. H+ secreted by H+-ATPase and H+-K+-ATPase combine with luminal NH3 to form NH4+, which is “trapped” in the lumen. In addition, there may also be minor components of diffusive NH3 movement across both the basolateral and apical plasma membranes (dotted lines). The intracellular H+ that is secreted by H+-ATPase and H+-K+-ATPase is generated by carbonic anhydrase (CA) II-accelerated CO2 hydration that forms carbonic acid, which dissociates to H+ and HCO3−. Basolateral Cl−/HCO3− exchange transports HCO3− across the basolateral membrane; HCO3− combines with H+ released from NH4+, to form carbonic acid, which dissociates to CO2 and water. This CO2 can recycle into the cell, supplying the CO2 used for cytosolic H+ production. The net result is NH4+ transport from the peritubular space into the luminal fluid. In the non-A, non-B cell, which lacks substantial basolateral Rhcg expression, Rhbg is likely the primary basolateral NH3 transport mechanism. The B-type intercalated cell, which lacks detectable Rhbg and Rhcg expression, likely mediates transcellular ammonia secretion through mechanisms only involving lipid-phase NH3 diffusion and thus transports ammonia at significantly slower rates.