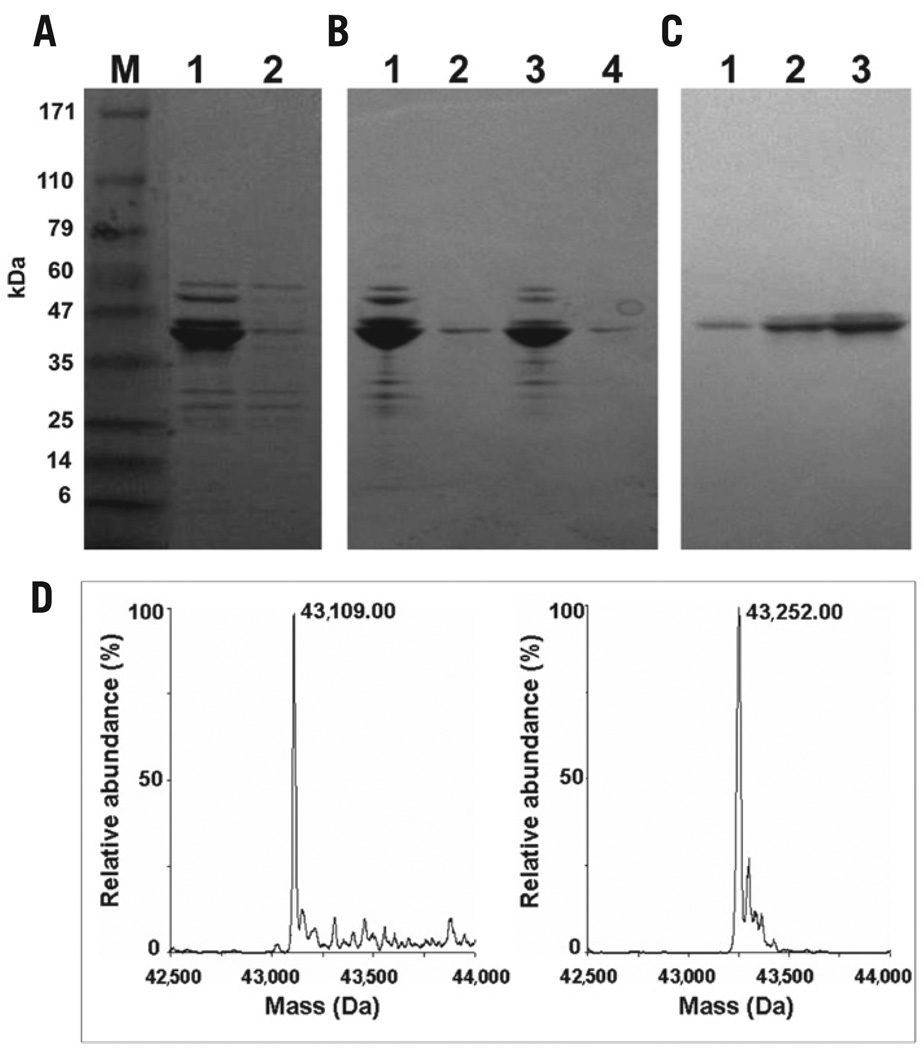
Figure 2. Expression and purification of MBP produced from E. coli [BL21-Codon Plus(DE3)-RIL] cells carrying pMAL-p2E.
(A) SDS-PAGE analysis of extracts (lane 1) from cells grown in the auto-induction medium and (lane 2) from cells grown in the same medium without lactate [noninduction medium MDAG (13)]. Lane M contains a mixture of molecular weight markers with masses indicated. (B) SDS-PAGE analysis of cell extracts: (lane 1) soluble fraction; (lane 2) insoluble fraction from cells grown on the auto-induction medium with glyphosate (1 g/L), amino acid mixture I (all 20 amino acids except Cys and Trp), and Trp (200 µg/L); (lane 3) soluble fraction; (lane 4) insoluble fraction from cells grown on the auto-induction medium with glyphosate (1 g/L), amino acid mixture I, 6F-Trp (316 mg/L), and Trp (50 µg/L). (C) SDS-PAGE of 6F-Trp-labeled MBP purified by affinity chromatography. Lanes 1, 2, and 3 show three sequential fractions eluted with 10 mM maltose. (D) Deconvoluted ESI-mass spectra of purified MBP obtained from cultures grown in a chemically defined auto-induction medium for aromatic unusual amino acid incorporation: (left) unlabeled MBP and (right) 6F-Trp-MBP.