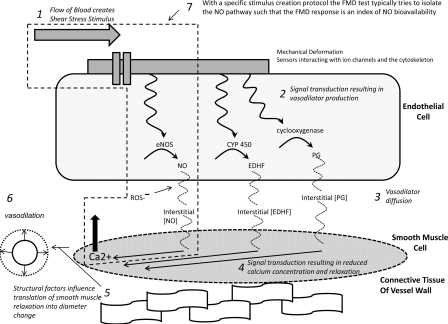
Fig. 1.
Schematic representation of steps involved in flow-mediated dilation (FMD) generation from the initiation of the shear-stress stimulus (step 1) to the resultant vessel diameter change (step 6). Blood flow-associated shear stress is sensed by deformation of mechanosensitive structures at the cell membrane. These structures could include the glycocalyx, the primary cilia, and mechanosensitive ion channels (19). Shear-stress mechanotransduction activates a signaling cascade that results in vasodilator production (step 2). The vasodilators produced and predominantly involved in FMD appear to depend on the nature of the shear-stress stimulus (100) and the endothelial phenotype (96). Vasodilators must diffuse from the endothelial cell into the smooth muscle cell (step 3). Nitric oxide (NO) may react with reactive oxygen species (ROS), decreasing its bioavailability (103). In the vascular smooth muscle, vasodilators trigger a signaling cascade that results in a lowering of calcium concentration and vasorelaxation (step 4). Vessel wall structural factors (e.g., relative proportions of collagen and elastin or wall-to-lumen ratio) may influence the diameter change that results from a given degree of smooth muscle relaxation (69) (step 5). FMD is quantified as the change in vessel diameter from baseline dimensions before the application of the shear-stress stimulus (step 6). Given the established vasoprotective properties of NO, FMD is often intended as an index of NO bioavailability (37, 100). Thus efforts have been directed at identifying specific protocols and stimulus profiles that are able to isolate the NO pathway (step 7). Examining vasodilatory responses to exogenous nitroglycerin can isolate and interrogate function downstream of the endothelium. EDHF, endothelial-derived hyperpolarizing factor; PG, vasodilatory prostaglandins; CYP 450, cytochrome P450; eNOS, endothelial NO synthase.