Abstract
Microtubules are composed of α-tubulin and β-tubulin dimers. Microtubules yield tubulin dimers when exposed to cold, which reassemble spontaneously to form microtubule fibers at 37°C. However, mammalian neurons, glial cells, and fibroblasts have cold-stable microtubules. While studying the microtubule toxicity mechanisms of the exotoxin Y from Pseudomonas aeruginosa in pulmonary microvascular endothelial cells, we observed that some endothelial microtubules were very difficult to disassemble in the cold. As a consequence, we designed studies to test the hypothesis that microvascular endothelium has a population of cold-stable microtubules. Pulmonary microvascular endothelial cells and HeLa cells (control) were grown under regular cell culture conditions, followed by exposure to an ice-cold water bath and a microtubule extraction protocol. Polymerized microtubules were detected by immunofluorescence confocal microscopy and Western blot analyses. After cold exposure, immunofluorescence revealed that the majority of HeLa cell microtubules disassembled, whereas a smaller population of endothelial cell microtubules disassembled. Immunoblot analyses showed that microvascular endothelial cells express the microtubule cold-stabilizing protein N-STOP (neuronal stable tubule-only polypeptides), and that N-STOP binds to endothelial microtubules after cold exposure, but not if microtubules are disassembled with nocodazole before cold exposure. Hence, pulmonary endothelia have a population of cold-stable microtubules.
Keywords: endothelium, stable tubule-only polypeptides
the endothelium forms the inner lining of all blood and lymphatics vessels. Due to this strategic localization, endothelium is a major regulator of numerous physiological functions, including blood coagulation, vascular tone, vascular permeability, angiogenesis, and leukocyte trafficking. Importantly, dynamic changes in endothelial shape are cardinal for some of these functions (19).
Endothelial cell shape is determined by dynamic adjustments between inward-directed, centripetal tension in contraposition to outward-directed, centrifugal traction, according to the environmental requirements (23). Actomyosin and actin-associated proteins are responsible for the centripetal forces, whereas microtubules, cell-cell, as well as cell-extracellular matrix adhesions, regulate the centrifugal pull (17). Microtubules are also key regulators of cell division and mitosis, as well as transport of vesicles and organelles throughout the cell (16). Due to their role in endothelial function, microtubules aid in engineering what is known as the semipermeable, nonthrombotic, anti-inflammatory, and vasodilatory nature of healthy endothelia, the paragon of cardiovascular health.
Microtubules are hollow structures that extend throughout the cell cytoplasm (28). Microtubules yield α- and β-tubulin dimers when exposed to cold (11). These dimers reassemble spontaneously to form microtubule fibers at 37°C in the presence of guanosine-5′-triphosphate. In contrast, microtubule networks of poikilotherms that live in the polar seas do not disassemble at very low temperatures, a phenomenon known as microtubule cold stability (29). Cold-stable microtubules also exist in plants and mammals. In the latter, pools of cold-stable microtubule networks have been identified in neurons, glial cells, and fibroblasts. In these cell types, cold stability is largely due to their association with a family of microtubule-associated proteins known as stable tubule-only polypeptides (STOP) (6).
While studying the microtubule toxicity mechanisms of the exotoxin Y from Pseudomonas aeruginosa in pulmonary microvascular endothelial cells (PMVECs), we observed that some endothelial microtubules were very difficult to disassemble in the cold. As a consequence of this observation, we designed studies to test the hypothesis that microvascular endothelium has a population of cold-stable microtubules. Here, we report that a majority of microvascular endothelial cell microtubules are indeed cold stable. Moreover, we show that this phenomenon is conserved across all endothelia in the pulmonary circulation, including the pulmonary artery endothelial cells (PAECs), PMVECs, and pulmonary vein endothelial cells (PVECs). In addition, we document that all lung endothelia express STOP, and that STOP binds to microtubules in response to cold. Some of these studies have been previously reported in the form of an abstract (19a).
MATERIAL AND METHODS
Cell culture.
PAECs (internal identification: PAECR16B); PMVECs (internal identification: PMVECR1); and PVECs (internal identification: PVECR16) were obtained from the Cell Culture Core at the University of South Alabama Center for Lung Biology. The isolation and characterization of these cells has been previously described in detail (1, 9, 15). HeLa cells were the kind gift of Dr. Jonathan Scammell (Department of Comparative Medicine, University of South Alabama), as well as purchased from ATCC (catalog no. CCL-2; Manassas, VA) and cultured in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum (catalog no. 10082; Invitrogen, Carslbad, CA) and 1% penicillin/streptomycin (catalog no. 15140; Invitrogen).
Cold exposure.
PMVECR1, PAEC16B, PVEC16, or HeLa cells were grown on glass coverslips (Thermo Fisher Scientific, Waltham, MA) or in six-well plates (Thermo Fisher Scientific) in complete Dulbecco's modified Eagle's medium until they reached 50–70% confluency. On the day of cold exposure, culture dishes were retrieved from the incubator and exposed to an ice-cold water bath (0°C) for 10 min on a bench top.
Immunofluorescence.
After cold exposure, cells were fixed either with or without permeabilization using a methanol (MeOH) fixation protocol. Briefly, cells were rinsed in phosphate-buffered saline (PBS), and they were plunged into −20°C 100% MeOH for 10 min in a −20°C freezer. After MeOH fixation, cells were rinsed in PBS, followed by either permeation using 0.1% Triton X-100 or regular immunofluorescence without permeation. Cells were blocked with 5% bovine serum albumin (BSA) for 10 min following permeation. Cells were then incubated with anti-β-tubulin antibody (catalog no. ab7291; Abcam, Cambridge, MA) or anti-α-tubulin monoclonal antibody (clone DM1A) (catalog no. T6199; Sigma-Aldrich, St. Louis, MO) for 1 h at room temperature. Cells were rinsed with PBS, followed by incubation with species-specific secondary antibodies (Alexa Fluor; Invitrogen) for 1 h. Cells were then rinsed with PBS and distilled water followed by mounting. Slides were viewed with a PerkinElmer Ultraview RS-3 spinning disk confocal microscope (Waltham, MA) (22). For some experiments, cold-treated cells were permeabilized before MeOH fixation. Permeabilization was performed by incubating cells on coverslips for 3 min in a buffer composed of 80 μM PIPES, pH 6.8, 1 mM EGTA, and 1 mM MgCl2 (PEM buffer), 0.5% Triton X-100, and 25% (wt/vol) glycerol.
Microtubule extraction protocol.
After cold exposure, cells were rinsed with PBS, and the buffer was removed. Next, 100 μl of PEM buffer containing 0.5% Triton X-100 and 25% glycerol were added to each dish to permeabilize the cells and release tubulin monomers (soluble tubulin). Paclitaxel (100 nM) replaced 25% glycerol for experiments in Fig. 8.
Fig. 8.
N-STOP associated with endothelial cell microtubules following exposure to paclitaxel. Top: Western blot analysis to detect N-STOP in polymerized vs. soluble tubulin fractions of PMVEC following treatment with 100 nM paclitaxel for 3 h. Bottom: Western blot analysis to detect α-tubulin in polymerized vs. soluble tubulin fractions of PMVEC following treatment with 100 nM paclitaxel for 3 h. Data are representative of 4 independent experiments.
After 3 min, the extraction buffer was removed, and the cells were rinsed with an additional 50 μl of buffer. The rinse buffer was collected and pooled with the initial extraction solution, and then 150 μl of 2× radioimmunoprecipitation assay (RIPA) buffer (catalog no. BP-115; Boston Bioproducts, Worcester, MA) with 1:100 protease inhibitors cocktail (catalog no. P8340; Sigma-Aldrich) was added to the pooled solution containing the soluble monomeric tubulin for a total volume of ∼300 μl. Next, cells ghosts (polymerized tubulin) were lysed with 300 μl of 1× RIPA buffer and protease inhibitor. Soluble and polymerized tubulin samples were stored at −80°C for future analyses.
Immunoblot analysis.
Cell lysates were generated by rinsing with cold (4°C) PBS followed by lysis with RIPA buffer (catalog no. BP-115; Boston Bioproducts) with 1:100 protease inhibitors cocktail (catalog no. P8340; Sigma-Aldrich). Cell lysates, soluble tubulin, and polymerized tubulin were normalized for protein concentration using the Lowry protein assay kit (procedure no. P5656; Sigma-Aldrich) and resolved in 4–12% Bis-Tris polyacrylamide gels (catalog no. NP0321; Invitrogen) and then transferred to 0.2-μm nitrocellulose membranes (catalog no. 162–0213; Bio-Rad, Hercules, CA). Membranes were incubated with appropriate antibodies. Neuronal STOP (N-STOP) expression was tested by using two monoclonal antibodies (catalog no. 4265; Cell Signaling, Danvers, MA, and catalog no. ab78077; Abcam) at 1:1,000 dilution in 5% BSA. Antibodies to α-tubulin (catalog no. ab7291; Abcam), and β-actin C4 (catalog no. sc-47778; Santa Cruz Biotechnology, Santa Cruz, CA) at 1:1,000 dilutions in 5% BSA were also used. Membranes were incubated with species-appropriate horseradish peroxidase-conjugated secondary antibody and developed using Supersignal West Femto chemiluminescent substrate (catalog no. 34096; Thermo Scientific, Rockford, IL). Western blot densitometries were measured using ImageJ software (National Institutes of Health, Bethesda, MD) by the method outlined at http://www.lukemiller.org/journal/2007/08/quantifying-western-blots-without.html and are expressed as area in arbitrary units.
Nocodazole treatment.
Endothelial cells were treated with nocodazole (10 μmol/l) for 90 min (30) before cold exposure and microtubule extraction.
Statistical analyses.
Data are presented as means ± SD. Polymerized and soluble tubulin were compared using Mann-Whitney test. Kruskal-Wallis statistic was used to compare N-STOP expression between PAEC, PVEC, and PMVEC. A value of P < 0.05 was considered statistically significant. GraphPad Prism 4.0 software (GraphPad Software, La Jolla, CA) was used for statistical analysis.
RESULTS
Cold exposure reveals two populations of microtubules in endothelial cells.
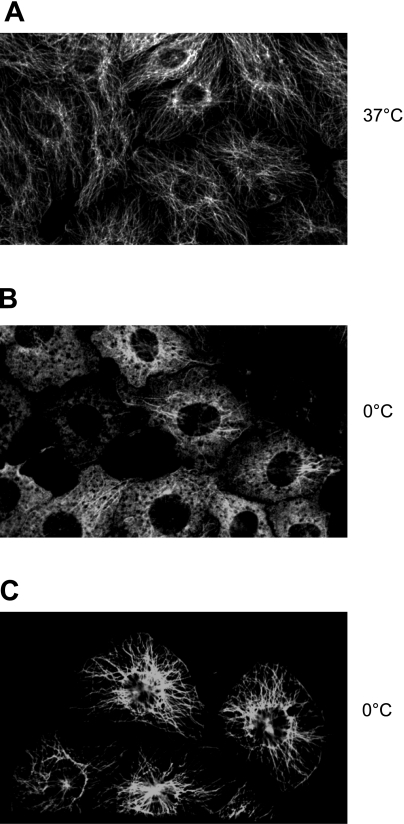
When endothelial cells were exposed to cold for 10 min, some microtubules disassembled, while others did not. The first population (cold unstable or cold sensitive) was revealed by the change in structure from microtubule fibers (Fig. 1A) to a fluorescent haze (Fig. 1B). This haze represents soluble tubulin from microtubules that disassembled in response to cold. This phenomenon is the typical response of microtubules to cold by most eukaryotic cells (see below). When endothelial cells are permeabilized, and a microtubule extraction protocol is used before immunofluorescence detection, microtubule fibers were detected in the remaining endothelial cells “ghosts” (Fig. 1C). These fibers are microtubules that are, therefore, cold stable or cold insensitive. We interpreted this finding to mean that endothelial cells have a population of cold-stable microtubules. Cold-stable microtubules were also detected in endothelial cells from the pulmonary artery and the pulmonary vein (data not shown).
Fig. 1.
Cold treatment uncovers two populations of microtubules in endothelial cells. A: endothelial cells have a vast array of microtubule fibers when cultured at 37°C. B: following exposure to cold for 10 min, some endothelial cell microtubules disassemble. These represent a group of endothelial cell microtubules that are cold sensitive. C: in addition, endothelial cells have a pool of cold-stable microtubules. This second microtubule population is only revealed if the soluble tubulin that disassembled with cold is extracted. Data are representative of 3 independent experiments.
HeLa cells do not have cold-stable microtubules.
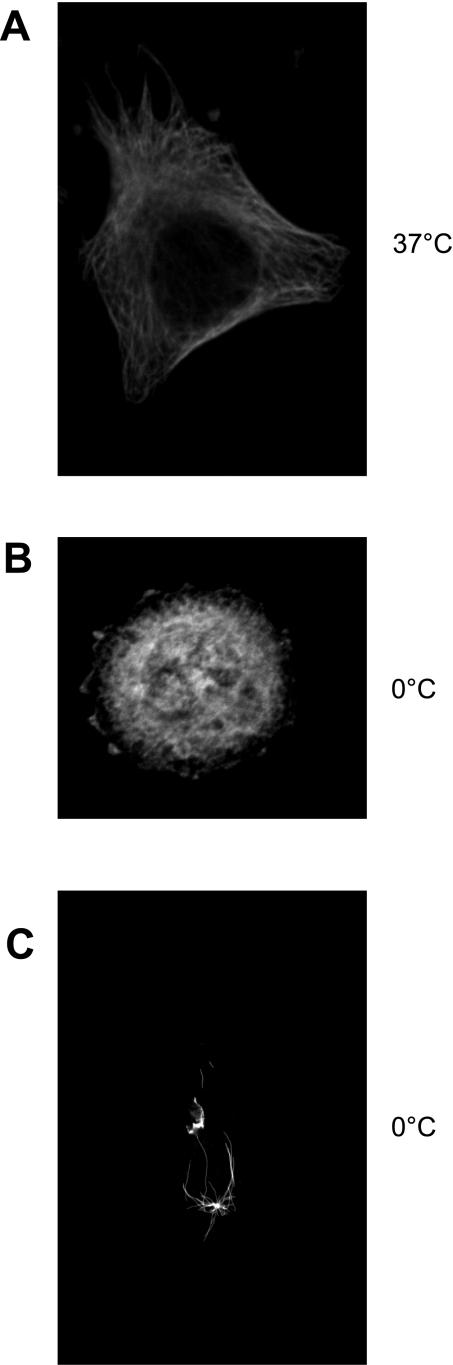
Disassembly following exposure to cold is considered the physiological response of polymerized tubulin. To verify this, HeLa cells were exposed to cold for 10 min, and most microtubules disassembled. This is demonstrated by a change from microtubule fibers (Fig. 2A) to a fluorescent haze (Fig. 2B), as well as by the change in HeLa cell shape from an elongated shape to a round shape. In contrast to what was found in endothelium, microtubule fibers were not detected with immunofluorescence following a microtubule extraction protocol (Fig. 2C). We interpreted this finding to mean that HeLa cells do not have cold-stable microtubules. This has been previously reported (10).
Fig. 2.
HeLa cells do not have cold-stable microtubules. A: immunofluorescence to detect α-tubulin in HeLa cells at 37°C. B: immunofluorescence to detect α-tubulin in HeLa cells following cold exposure for 10 min. Soluble tubulin was not extracted. C: immunofluorescence to detect α-tubulin in HeLa cells after cold exposure and tubulin extraction. Data are representative of 3 different experiments.
Western blot analyses confirm cold-stable microtubules in endothelial cells.
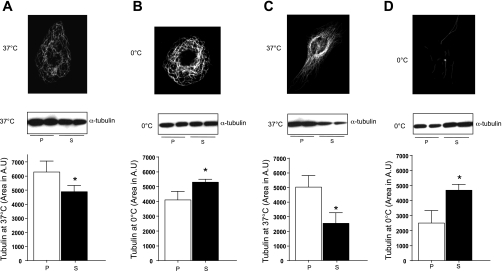
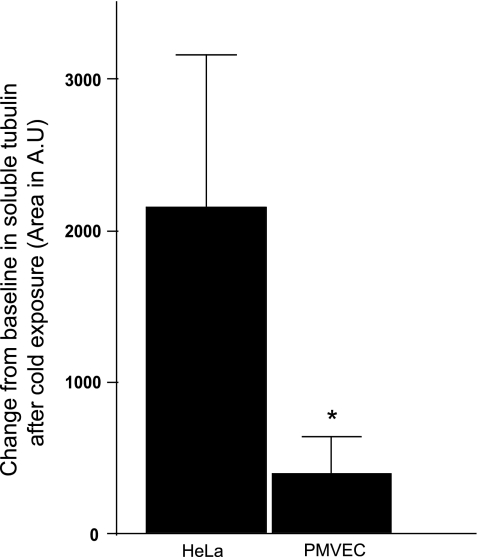
Western blot to detect α-tubulin reveals that, at 37°C, endothelial cells have more polymerized than soluble tubulin (Fig. 3A). This relation changes following exposure to 0°C (Fig. 3B). Cold treatment did, in fact, disassemble some endothelial cells microtubules, as the soluble tubulin fraction increased compared with the polymerized fraction (Fig. 3B). In HeLa cells, the fraction of soluble tubulin increased even more, compared with endothelium, following cold treatment (Fig. 3, C and D). When the change from baseline in soluble tubulin following cold exposure was evaluated in HeLa vs. endothelial cells, HeLa cells demonstrated a threefold increase in soluble tubulin compared with endothelial cells (Fig. 4). These observations were confirmed using paclitaxel in the extraction buffer (see supplemental material; available online at the AJP-Lung Cellular and Molecular Physiology web site).
Fig. 3.
Microtubules and α-tubulin in microvascular endothelium vs. HeLa cells. A: at 37°C, pulmonary microvascular endothelial cells (PMVEC) have more polymerized than soluble tubulin. Top: immunofluorescence to detect α-tubulin in PMVEC cells at 37°C. Picture is representative of 6 independent experiments. Middle: immunoblot to detect polymerized (P) vs. soluble (S) α-tubulin following microtubule extraction at 37°C. Bottom: quantitation of immunoblot results. Data are representative of 5 independent experiments. B: following cold exposure, PMVEC have more soluble than polymerized tubulin; however, the change is not as dramatic as in HeLa cells. Top: immunofluorescence to detect α-tubulin in PMVEC at 0°C. Picture is representative of 6 independent experiments. Middle: immunoblot to detect polymerized vs. soluble α-tubulin following microtubule extraction. Bottom: quantitation of immunoblot results. Data are representative of 5 independent experiments. C: at 37°C, HeLa cells have more polymerized than soluble tubulin. Top: immunofluorescence to detect α-tubulin in HeLa cells at 37°C. Picture is representative of 6 independent experiments. Middle: immunoblot to detect polymerized vs. soluble α-tubulin following microtubule extraction. Bottom: data are representative of 5 independent experiments. D: following cold exposure, HeLa cells have more soluble than polymerized tubulin. Top: immunofluorescence to detect α-tubulin in HeLa cells at 0°C. Picture is representative of 8 independent experiments. Middle: immunoblot to detect polymerized vs. depolymerized α-tubulin following microtubule extraction. Bottom: quantitation of immunoblot results. Bottom values are means ± SD. Data are representative of 5 independent experiments. *P < 0.05. Statistical significance was determined by Mann-Whitney test.
Fig. 4.
Change in extracted α-tubulin following cold exposure. Change from baseline in extracted tubulin was estimated by subtracting the area of soluble α-tubulin calculated by densitometry of Western blot analysis at 37°C from area of soluble α-tubulin calculated by densitometry of Western blot analysis at 0°C, in both HeLa cells and PMVEC. Values are means ± SD. *P < 0.05. Statistical significance was determined by Mann-Whitney test.
Time course of cold treatment in endothelial cells.
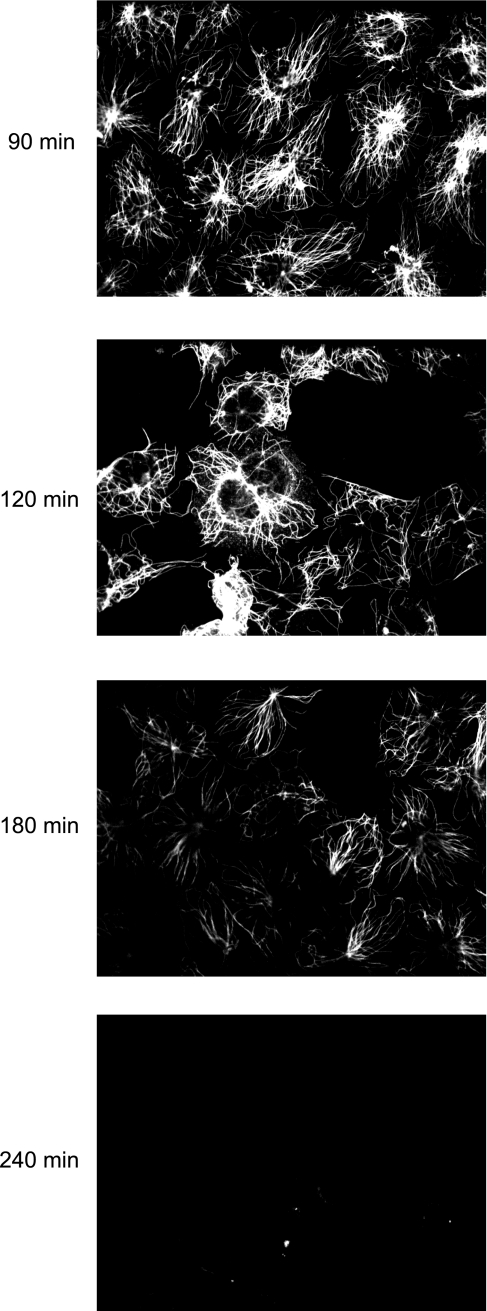
While the majority of HeLa cell microtubules disassembled by 10 min after exposure to cold, microtubule fibers were detected in endothelial cells up to 180 min following cold treatment (Fig. 5). These microtubules eventually depolymerized and were undetectable after 240 min of exposure to 0°C.
Fig. 5.
Endothelial cell microtubules are detected up to 180 min of cold treatment. A time course of cold treatment shows that endothelial microtubule fibers are detected after 90, 120, and 180 min of cold exposure. This is in contrast to HeLa cells in which microtubules disassembled within 10 min of treatment with cold.
Endothelial cells express N-STOP.
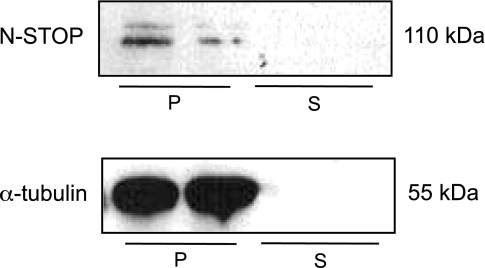
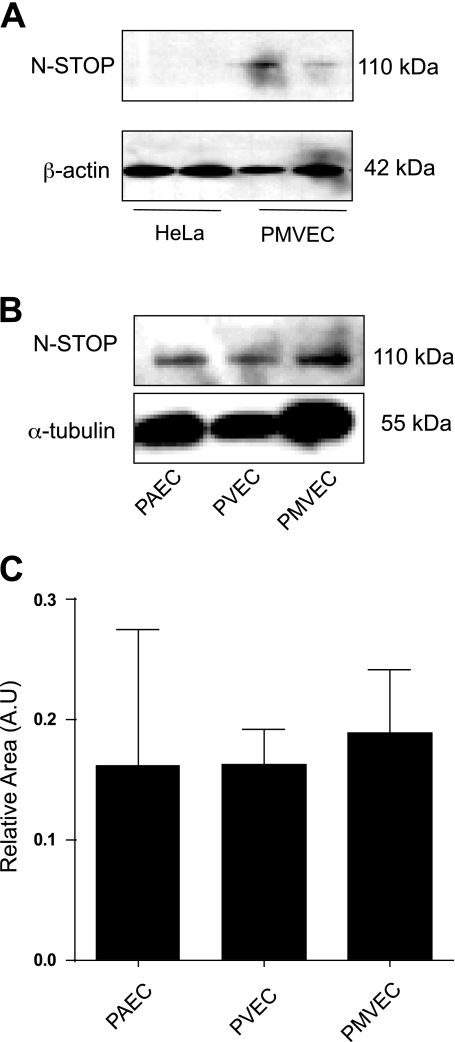
Western blot analyses on cell lysates showed that PMVEC express N-STOP, while HeLa cells do not (Fig. 6A). HeLa cells are devoid of N-STOP expression (10). N-STOP was expressed across all types of the endothelial cells lining the pulmonary circulation (Fig. 6B), with no statistical difference in level of expression among the three endothelia (Fig. 6C).
Fig. 6.
Endothelial cells express neuronal stable tubule-only polypeptides (N-STOP). A: N-STOP (110 kDa) is expressed in PMVEC (right) but not in HeLa cells (left). B: in addition, N-STOP is expressed in pulmonary artery endothelial cells (PAEC), as well as pulmonary vein endothelial cells (PVEC). C: data are representative of 4 independent experiments. Relative area is the ratio of the area of N-STOP measured by densitometry of Western blot analysis and the area of α-tubulin measured by densitometry of Western blot analysis. Values are means ± SD. Statistical significance was evaluated using one-way ANOVA (Kruskal-Wallis statistic; P = 0.9260).
N-STOP associated with endothelial cell microtubules following exposure to cold.
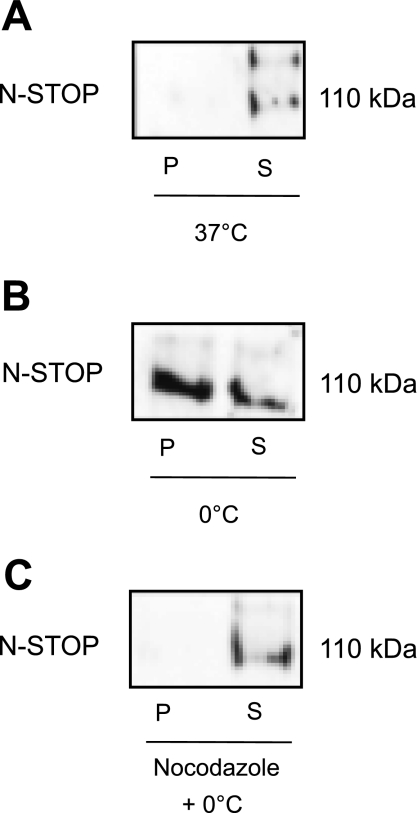
Western blot analysis revealed that N-STOP is extracted with the soluble fraction of microtubules, indicating that N-STOP does not associate with polymerized microtubules under control conditions at 37°C (Fig. 7A). However, after cold treatment, N-STOP was detected in the polymerized tubulin fraction, as well as the soluble fraction (Fig. 7B). This suggests that, following cold exposure, N-STOP associates with polymerized microtubules. Finally, when microtubules were disassembled using nocodazole before exposure to 0°C, N-STOP was not detected in the polymerized fraction, but in the soluble tubulin fraction (Fig. 7C).
Fig. 7.
N-STOP binds to polymerized tubulin following cold exposure. A: at 37°C, N-STOP is found in the soluble tubulin fraction. B: following cold exposure, N-STOP is found in both the polymerized and soluble fraction. C: when microtubules were depolymerized before cold exposure, N-STOP was found in the soluble fraction.
N-STOP associated with endothelial cell microtubules following exposure to paclitaxel.
While in neurons, cold-stable microtubules are also resistant to disassembly by nocodazole; in endothelium, nocodazole disassembled all microtubules, including those that are cold stable. To test whether this difference resulted from the rapid microtubule turnover rate seen in endothelial cells vs. neurons, we exposed endothelial cells to paclitaxel, followed by detection of N-STOP by Western blot analysis. We found that N-STOP appeared in the polymerized tubulin fraction following paclitaxel treatment for 3 h (Fig. 8). We interpreted this to mean that N-STOP is removed earlier in the microtubule disassembly process in endothelium compared with neuronal microtubules.
DISCUSSION
The studies that defined the role of microtubules in regulating cell shape relied on the ability of low temperature to induce microtubule breakdown (26). However, not all mammalian microtubules are cold sensitive (29). Cold (<15°C) stability in microtubules is recognized in neurons, glial cells, and fibroblasts (6). Our report describes, for the first time, a population of cold-stable microtubules in pulmonary endothelial cells.
Our studies revealed that cold stability is a phenomenon conserved across endothelial cells from all vascular beds of the pulmonary circulation. This is an important observation, since it is widely accepted, both in vitro and in vivo, that endothelial cells from the same organ system are heterogeneous in nature. In the pulmonary circulation, for example, endothelial cells lining the blood vessels with a diameter >25 μm are morphologically and functionally different than those endothelial cells lining the capillaries. They have different gene expression patterns (7), lectin binding capacity (15), resting membrane potentials (25), intracellular calcium (8) and cAMP dynamics (24), hydraulic conductance (21), caveolar density (19), and even vasculogenic capacity (1). The fact that all pulmonary endothelia have a pool of cold-stable microtubules brings into question how these microtubule pools are differentially regulated. Future studies need to systematically address this question, especially since recent evidence shows that endothelial microfilament stability is different among phenotypes, given that there is a fivefold greater cytochalasin D concentration required to disrupt the actin cytoskeleton of lung microvascular endothelial cells compared with lung arterial endothelial cells (20).
In mammals, microtubule cold stability is predominantly due to a family of microtubule-associated proteins known as stable tubule-only polypeptides, or STOP (6). STOP proteins are calmodulin-regulated proteins that induce resistance to cold and nocodazole by binding directly to microtubules. The first STOP was isolated from adult neurons and named N-STOP (110–145 kDa) (18). Subsequently, two splice variants were reported and termed E-STOP (for early STOP, 84 kDa) (13) and F-STOP (for fibroblastic STOP, 42 kDa) (10). Recently, two other isoforms of STOP were identified in astrocytes and oligodendrocytes and named A-STOP (60 kDa) and O-STOP (89 kDa), respectively (12). In the present report, studies were performed to assess whether microvascular endothelial cells expressed STOP proteins. Using two different monoclonal antibodies, these studies detected the N-STOP variant at ∼110 kDa.
The distinct STOP isoforms interact with microtubules differently under physiological conditions. These differences have been associated with the rapidity of microtubule turnover (6). In neurons, where microtubule turnover is low, N-STOP and E-STOP associate constitutively with microtubules. In contrast, in fibroblasts, where microtubule turnover is high, F-STOP is not constitutively associated with microtubule fibers, but interacts with them within seconds following cold exposure (10). Our studies showed that, in microvascular endothelium, N-STOP was found in soluble cell fractions at 37°C. However, following cold exposure, N-STOP was detected in both insoluble and soluble fractions. These observations were interpreted to mean that, at 37°C, endothelial N-STOP is found soluble in the cytoplasm, but that, following cold exposure, N-STOP associates with microtubules. This interpretation is supported by the observation that, when endothelial cells are treated with nocodazole immediately before cold exposure, N-STOP appears in the soluble fractions.
The independent contribution of this pool of cold-stable microtubules to endothelial physiology in general, and to endothelial barrier properties in particular, is unclear. Microtubules are critical in the function of endothelium as a semipermeable barrier. Microtubules, in association with cell-cell as well as cell-matrix adhesions, generate a centrifugal force that resists the centripetal compression created by actomyosin and actin-associated proteins (14). The physiological importance of the microtubule contribution to this centrifugal vector is underscored in the tight vascular barrier that the pulmonary microvascular endothelium forms to allow proper gas exchange. When this barrier is disrupted, inflammatory alveolar edema occurs, and gas exchange is compromised. Investigations have shown that, when PMVEC microtubules are disassembled, via nocodazole, thrombin, or bacterial exotoxins, interendothelial gaps form and endothelial cell permeability increases (2–5, 22, 27). Therefore, future studies aimed at addressing the role of cold-stable microtubules in endothelial permeability are critical. In addition, investigations addressing whether cold-stable microtubules contribute specifically to the endothelial intracellular homeostasis, like organelle and macromolecular transport, cell division, and tensional integrity, are needed.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-60024 and HL-66299 (T. Stevens and R. Balczon) and HL-76125 (C. D. Ochoa).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Linn Ayers and Anna Buford for their contribution to the development of this work.
REFERENCES
- 1. Alvarez DF, Huang L, King JA, El Zarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Birukova A. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 289: L75–L84, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Birukova A. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Birukova A. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 287: L86–L93, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bosc C, Andrieux A, Job D. STOP proteins. Biochemistry 42: 12125–32, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A 100: 10623–10628, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267–1278, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Creighton J, Zhu B, Alexeyev M, Stevens T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J Cell Sci 121: 110–119, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Denarier E, Fourest-Lieuvin A, Bosc C, Pirollet F, Chapel A, Margolis RL, Job D. Nonneuronal isoforms of STOP protein are responsible for microtubule cold stability in mammalian fibroblasts. Proc Natl Acad Sci USA 95: 6055–6060, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Detrich HW, 3rd, Parker SK, Williams RC, Jr, Nogales E, Downing KH. Cold adaptation of microtubule assembly and dynamics. Structural interpretation of primary sequence changes present in the alpha- and beta-tubulins of Antarctic fishes. J Biol Chem 275: 37038–37047, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Galiano MR, Bosc C, Schweitzer A, Andrieux A, Job D, Hallak ME. Astrocytes and oligodendrocytes express different STOP protein isoforms. J Neurosci Res 78: 329–337, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Guillaud L, Bosc C, Fourest-Lieuvin A, Denarier E, Pirollet F, Lafanechãre L, Job D. STOP proteins are responsible for the high degree of microtubule stabilization observed in neuronal cells. J Cell Biol 142: 167–79, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ingber DE. From cellular mechanotransduction to biologically inspired engineering: 2009 Pritzker Award Lecture, BMES Annual Meeting October 10, 2009. Ann Biomed Eng 38: 1148–1161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kueh HY, Mitchison TJ. Structural plasticity in actin and tubulin polymer dynamics. Science 325: 960–963, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee TY, Gotlieb AI. Microfilaments and microtubules maintain endothelial integrity. Microsc Res Tech 60: 115–127, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Margolis RL, Rauch CT, Job D. Purification and assay of a 145-kDa protein (STOP145) with microtubule-stabilizing and motility behavior. Proc Natl Acad Sci U S A 83: 639–643, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 19a. Ochoa CD, Stevens T, Balczon R. Freezing temperatures do not disassemble endothelial cell microtubules (Abstract). Am J Respir Crit Care Med 181: A3413, 2010 [Google Scholar]
- 20. Ofori-Acquah SF, King J, Voelkel N, Schaphorst KL, Stevens T. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res 75: 391–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parker JC, Stevens T, Randall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 291: L30–L37, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol 297: L73–L83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res 77: 53–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol Lung Cell Mol Physiol 277: L119–L126, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Stevens T, Fouty B, Hepler L, Richardson D, Brough G, McMurtry IF, Rodman DM. Cytosolic Ca2+ and adenylyl cyclase responses in phenotypically distinct pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 272: L51–L59, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Tilney LG, Porter KR. Studies on the microtubules in heliozoa. II. The effect of low temperature on these structures in the formation and maintenance of the axopodia. J Cell Biol 34: 327–343, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol 281: L565–L574, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Wade RH. Microtubules: an overview. Methods Mol Med 137: 1–16, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Wallin M, Stromberg E. Cold-stable and cold-adapted microtubules. Int Rev Cytol 157: 1–31, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Wu S, Chen H, Alexeyev MF, King JA, Moore TM, Stevens T, Balczon RD. Microtubule motors regulate ISOC activation necessary to increase endothelial cell permeability. J Biol Chem 282: 34801–34808, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.