Abstract
In this study, we characterized the patterns and timing of cortical activation of visually guided movements in a task with critical temporal demands. In particular, we investigated the neural correlates of motor planning and on-line adjustments of reaching movements in a choice-reaction time task. High-density electroencephalograohy (EEG, 256 electrodes) was recorded in 13 subjects performing reaching movements. The topography of the movement-related spectral perturbation was established across five 250-ms temporal windows (from prestimulus to postmovement) and five frequency bands (from theta to beta). Nine regions of interest were then identified on the scalp, and their activity was correlated with specific behavioral outcomes reflecting motor planning and on-line adjustments. Phase coherence analysis was performed between selected sites. We found that motor planning and on-line adjustments share similar topography in a fronto-parietal network, involving mostly low frequency bands. In addition, activities in the high and low frequency ranges have differential function in the modulation of attention with the former reflecting the prestimulus, top-down processes needed to promote timely responses, and the latter the planning and control of sensory-motor processes.
INTRODUCTION
Movements aimed to a target, even the rapid ones, result from an initial, preplanned phase and from adjustments implemented during the execution (Woodworth 1899). Shifts from the original trajectory plan are most obvious in response to external perturbations, such as changes in target position. In the absence of perturbation, adjustments are seen as deviations of peak acceleration from the linear scaling to movement amplitude: for example, in the case of planar reaching movements, the movement amplitude is not completely predicted by the peak acceleration, i.e., its second-time derivative that reflects planning (Ghez and Gordon 1987; Gordon et al. 1994a,b). On-line changes of the initial planning are prepared and implemented during the unfolding of the motor program through the modulation of the movement duration (Ghez and Gordon 1987; Gordon et al. 1994a,b; Higgins and Angel 1970; Vicario and Ghez 1984). The advanced preparation processes, or feedforward mechanisms, and those involved in overriding a motor plan in progress, or feedback mechanisms, rely on the integrity of sensory and memory systems and thus are based on the processing of sensory information as well as on the access and update of internal models.
To dissect the neural bases of the different mechanisms involved in reaching, electrophysiological and imaging studies have employed a variety of experimental paradigms in both animals and humans (Battaglia-Mayer et al. 2003; Culham and Valyear 2006). In general, reaching-related activations involve a diffuse fronto-parietal network that includes the dorsal occipital and parietal cortices, the premotor, motor, supplementary areas (SMA), and cingulate cortex (ACC) (Grafton et al. 1996). The temporal stream of activations of these areas is still a matter of debate (Bernier et al. 2009; Grafton et al. 1996; Naranjo et al. 2007), and the functional bases of the processes implicated in both movement planning and changes of motor plans have been only partially uncovered. On one hand, advanced motor planning and sensory-motor information processing that relies on feedforward mechanisms involve especially posterior parietal and premotor cortices, as shown by monkey and human studies (Andersen and Buneo 2002; Batista et al. 1999; Hoshi and Tanji 2004; Medendorp et al. 2007), and the midline frontal regions, as reported by human investigations (Tombini et al. 2009). On the other hand, the results of the few studies on the neural correlates of the feedback processes that are involved in the trajectory changes following external perturbations suggest that those same areas are involved in movement corrections (Desmurget et al. 1999; Pisella et al. 2000).
In humans, motor-related activation has been successfully investigated with the analysis of electroencephalographic (EEG) oscillatory activity. However, only a few investigations have employed this approach to study reaching, catching or grasping (Tombini et al. 2009; Van Der Werf et al. 2010; Virji-Babul et al. 2010). The patterns of induced oscillatory activity in these studies are similar to those reported with simpler motor paradigms in terms of both frequency range and topography (Neuper and Pfurtscheller 2001): in all, before the onset and during the movement, a power decrease in the alpha (8–14 Hz) and beta bands (15–30 Hz) occurs bilaterally over the sensory-motor and parietal sites and in the frontal midline regions. Of note, in reaching and catching, posterior and frontal sites show a greater activation, probably due to more complex visuomotor transformation and attention modulation (Senot et al. 2008; Tombini et al. 2009). In addition, synchronization of frontal theta power is found during the planning phase of catching in a learning paradigm with theta activity decreasing as a function of expertise (Tombini et al. 2009).
All of the mentioned studies have focused only on one or a few aspects of the processes involved in movement planning or execution, using tasks that produced a parcellation of such processes. Single responses occurred with long time intervals, sometimes perturbed visually or by transient lesions of brain activity, situations that are relatively far from ecological conditions and contexts. In the present study, we wished to characterize the pattern and the timing of the cortical activation related to visually guided movements in a choice-reaction time paradigm with a task without external perturbations but with significant temporal demands: stimuli were presented at a fast rate and responses had to be completed in a short time period. This task more closely simulates many of our daily-life situations in which movements occur in a continuum rather than being separated by long resting intervals. We hypothesize that, in such a task, motor planning and on-line adjustments in part share frequency bands and topography in a fronto-parietal network. In addition, we postulate that the attentional requirements for movement preparation and execution are reflected by involvement in different frequency bands. Therefore we studied the oscillatory power variation and phase coherence between selected scalp sites. Moreover, we estimated correlations between regional, time-dependent EEG oscillations and specific behavioral indices known to reflect distinct processes of reaching. This correlation approach, which is based on the assumption that regional physiology varies systematically with the magnitude of cognitive or sensorimotor processes, provides important information about the dynamics of complex behavior and has been successfully used EEG studies (Bradberry et al. 2010; Huber et al. 2004).
The results of the current investigation show that visually guided movements in a choice-reaction time paradigm result from the combination of a preplanned phase and by subsequent adjustments, like for reaching movements with advanced information (Ghez and Gordon 1987; Gordon et al. 1994a,b). More importantly, they demonstrate that preplanning and adjustment phases show a partial dissociation in terms of regional EEG oscillations and frequency domains.
METHODS
Subjects
Thirteen right-handed naive subjects [age: 22 ± 1 (SD) yr; 11 men and 2 women] participated in the study. They all had normal or corrected vision and no history of neurological and psychiatric disorders. Written informed consent was obtained from all participants and the experiments were conducted with the approval of the CCNY Institutional Review Board.
Experimental setup
Subjects were seated in front of a computer monitor and moved a cursor on a horizontal digitizing tablet with their right hand (sampling rate, 200 Hz). Hd-EEG was recorded during the whole experiment. An opaque panel placed at shoulder level prevented the view of the hand and forearm at all times.
Motor task
General features of the motor task are described in details in previous studies (Ghilardi et al. 2003, 2009; Moisello et al. 2009). Briefly, subjects were required to perform straight out and back movements from a central starting point to one of eight radially arrayed targets (distance, 4.0 cm; Fig. 1A) displayed as circles on a screen. Targets (stimuli) were presented in a pseudo-random, nonrepeating order, at 1.5 s intervals. A “block” consisted of 88 movements, for a total duration of 132 s. Importantly, all subjects were able to complete each movement before the appearance of the following target. This was achieved in a few movements (from 2 to 10) in a preceding familiarization session.
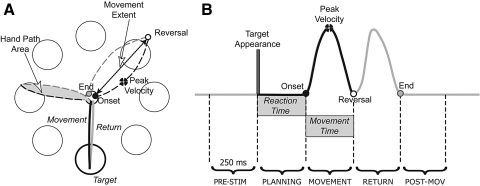
Fig. 1.
A: target array with 3 examples of trajectory (back- forward movement, gray- return). Representation of hand path area, movement extent, peak velocity and the points of onset, reversal, and end. B: velocity profile of a representative out and back movement with the 5 temporal windows used in the electroencephalographic (EEG) analysis.
Subjects were instructed to move as fast and as accurately as possible, without correction, and to reverse sharply within the target circle (diameter: 2 cm). As reported in previous publications (Ghilardi et al. 2000, 2003), several spatial and temporal indices were measured for each movement (see also Fig. 1, A and B). In particular, we computed: reaction time: the time from the target appearance (stimulus presentation) to the movement onset; amplitudes of peak velocity and peak acceleration; movement time: the time from movement onset to reversal; movement extent or amplitude: the length of the segment (straight shortest distance) from onset to reversal point; normalized area: the area enclosed by the hand path divided by the square of movement length (Moisello et al. 2008); and curvature: the difference of the orientation of the vector from the movement starting point through the point of the peak velocity and that of the vector from the movement start through the endpoint.
To study the brain dynamics related to movement preparation and execution, in addition to the onset time, for all movements, we calculated the latencies of the reversal point, the return to the center and the presentation of the stimulus (Fig. 1B). These events were then aligned to the EEG recordings and further used for signal processing as detailed in the following paragraphs.
EEG recording and signal processing
High-density EEG data were recorded from 256 electrodes (Hydrocel net, Electrical Geodesics) while subjects performed the motor tasks. The electrodes' net was applied on subjects' scalp in accordance with the direction specified in the net manual (Hydrocel net, Electrical Geodesics). During the recording, the EEG signal was referenced to a Cz sensor. Data were collected using the high-impedance amplifier Net Amp 300 and Net Station 4.3 (Electrical Geodesics). Impedances were kept <50 kΩ with a sampling rate of 250 Hz.
The EEG data of two subjects were excluded from the analysis due to technical problems. In the remaining 11 subjects, after the exclusion of the channels located on the neck and cheeks, 183 site scalp electrodes were selected and used for further analysis. A linked mastoid reference montage was used in the principal component analysis (PCA); for all the remaining analyses, data were re-referenced to the average across the 183 electrodes.
PREPROCESSING.
A preprocessing procedure was performed on the continuous data with NetStation 4.3 software (Electrical Geodesics) and the public license toolbox EEGLAB (Delorme and Makeig 2004). In the first step, the continuous EEG signal was high-pass filtered >0.5 Hz and low-pass filtered <80 Hz. To filter out the line noise, we adopted a notch filtered centered at 60 Hz. Afterwards channels affected by bad scalp-electrode contact were visually identified and further replaced with data interpolation from all the remaining electrodes (spherical splines interpolation was used). After that, the continuous EEG signal was segmented into epochs of 1.5 s based on movement onset latencies (from −700 to 800 ms after the movement onset). Visual rejection of bad epochs followed. It consisted in the identification and rejection of epochs contaminated by nonstereotyped artifacts such as burst movements (like coughing or sneezing) or huge contractions of head and neck muscles. It is important to note that stereotyped artifacts like blinks, eye movements, and motion related signals were kept in the epoched files to be estimated and removed by PCA technique. Preprocessed EEG data were then imported into EEGLAB for further analysis.
To identify and remove the extra-brain artifactual stereotyped sources from the EEG signal, PCA was applied on a single-trial basis (Dien and Frishkoff 2005). To get reliable components, for each subject, the raw EEG signal recorded during the 14 blocks was concatenated and the resulting file submitted to the PCA to derive 100 linearly independent spatial filters. The decomposition derived from the calculation was then applied to each block file. The PCA-processed EEG data were then visually inspected and the components accounting for the eye, muscle, motion related and other kind of artifacts (e.g., line noise) were removed.
Because the main interest in the current investigation was to characterize the brain dynamics related to target reaching movements, we focused on the first two blocks of the motor task in which no visuo-motor transformation was applied to the cursor. Following the preprocessing and the PCA cleaning procedures, an average number of 139 ± 15 of the initial 188 trials was used. All the results described in this paper were hence carried on and derived specifically from these two blocks.
EVENT RELATED SPECTRAL PERTURBATION AND THE IDENTIFICATION OF SCALP REGIONS OF INTEREST.
After merging the PCA pruned EEG signals from the two blocks, for each subject, we estimated individual event related spectral perturbation (ERSP) by measuring the mean event-related changes in the power spectrum at all the channels over time with respect to a baseline period. To compute the changes in power spectrum, each epoch was submitted to short-time Fourier analysis using fast Fourier transforms (FFTs). Each trial of 375 sample points (1.5 s) was segmented in 92 segments that were individually multiplied by a Hanning window. The windows were 128 data points (516 ms) long with 96% overlap. We obtained amplitude estimates in the frequency range between 2 and 35 Hz, and the distance between two adjacent bins was 0.25 Hz (for a detailed description of the adopted method, see Delorme and Makeig 2004). The selection of the baseline period was made with the intent to bolster the brain activity patterns occurring in the preparation and the execution of the reaching movements and with the goal to tap a period in which the subjects were motionless. To do so, for each subject, we estimated the mean latencies of the return to center and the mean time of stimulus presentation. On the basis of these two variables, we determined the individual time window in which the subjects were completely motionless. Baseline period was set to last for 300 ms and end 250 ms before the target appearance. However, due to the very narrow inter-trial interval (1.5), we expected that the selection of the baseline might sensibly affect the estimation of power perturbation. Hence we carried on the same analysis on the same set of data using the mean power across the entire epoch (1.5 s) as a baseline. Results from this parallel procedure will be presented in supplementary materials and the main differences with the short epoch baseline analysis discussed.
Following the time/frequency decomposition, individual ERSPs were averaged across specific time windows and frequency ranges. We were interested in estimating the power perturbation associated with specific stages of planning and execution of the movements. Hence, for each subject, five time windows (Fig. 1B) were selected on the basis of his/her behavioral data. As for the identification of the baseline, individual mean latencies of stimulus presentation, onset and return to center were used. The time intervals were defined as in the following: PreStim: from 250 ms before stimulus presentation to stimulus presentation; planning: from 250 ms before movement onset to movement onset; movement: from movement onset to 250 ms; return: from 250 ms before the return to center to the return to center; PostMov, from the return to center to 250 ms after.
The following frequency ranges were selected: theta (4.15–7.81 Hz), alpha-1 (8.05–10 Hz), alpha-2 (10.25–11.96), beta-1 (12.20–17.82), beta-2 (18.06–24.9).
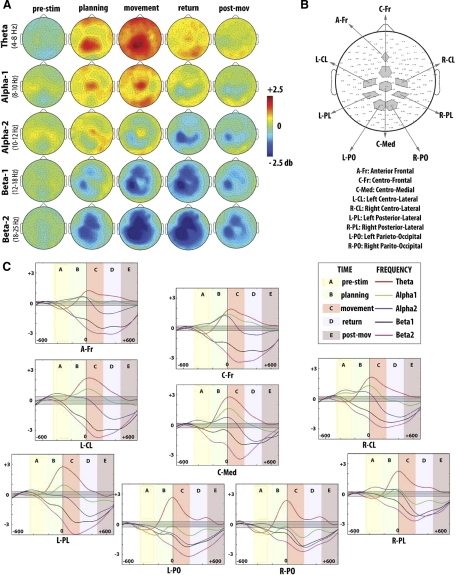
The mean individual ERSP amplitudes for these time intervals and frequency bands were averaged across subjects and then plotted on the scalp to identify set of electrodes showing intensive power perturbation. Because the aim of this study was to determine the neural bases of feedforward and -back mechanisms, we focused on those time windows overlapping the preparation and execution of the movements (PreStim, planning, movement, and return). To get n scalp regions of interests (S-ROIs) surrounding the fronto-parietal network of reaching, for each time intervals and frequency bands, the “responsive electrodes” were identified on the basis of the group-mean topographical maps obtained from the spectral analysis and reported in Fig. 2A. Of note, the reliability of power perturbation of the selected electrodes was further confirmed with statistical procedure. A detailed description of S-ROIs identification procedure is provided in the supplemental material.1
Fig. 2.
Findings obtained in the event related spectral perturbation (ERSP) analysis. A: group-mean ERSP for the 5 selected frequency bands are plotted on the scalp at the different time intervals of interest. B: the 9 scalp regions of interest (S-ROIs) that were selected on the basis of the ERSP activation patterns. The S-ROIs are depicted in gray along with the corresponding electrodes. C: time course of the ERSP calculated on the group for the 9 scalp S-ROIs and significance limits (in gray) obtained with bootstrap statistic (P < 0.01).
Nine S-ROIs were identified: two sets of six electrodes each overlapping the left and right centro-lateral scalp regions (L-CL and R-CL); two sets of six electrodes each corresponding to posterior lateral scalp regions (L-PL and R-PL); two sets of seven electrodes each located on the left and right medial parieto-occipital regions (L-PO and R-PO); a set of four electrodes located on the anterior frontal scalp region (A-Fr); a set of six electrodes surrounding the centro-frontal region (C-Fr); a set of seven electrodes overlapping the centro-medial region (C-Med). Figure 2B shows the selected S-ROIs. The EEG raw signal of the electrodes within each S-ROI was then averaged for further analysis.
Group and individual S-ROIs spectral activities were computed through short-time Fourier analysis. The adopted procedure was the same as that previously described for the individual time/frequency decomposition at channel level.
As for the group, to test with bootstrap procedure (P = 0.01) the changes of power at any time of the epoch with respect to the baseline period, we first concatenated the S-ROI individual raw signals and then performed a ERSP analysis on the resulting file (Delorme and Makeig 2004). The baseline-normalized power was further averaged across bins within the selected frequency bands to have S-ROIs time course plots.
As for the investigation of the functional contribution of S-ROIs activity to motor performance, we determined the individual ERSP of each S-ROIs in selected time windows and frequencies and further correlated them with behavioral indices. In detail, the EEG raw signal of each subject recorded at the selected sensors was first averaged within each S-ROI and then submitted to short-time Fourier analysis (for the details, see preceding text). The resulting individual power estimates were averaged within the preparation, planning and out-reaching time windows (PreStim, planning, movement) and frequency bands as previously described. The resulting individual S-ROIs mean ERSPs were then correlated (Pearson's correlation) with kinematic indices within the group.
EVENT RELATED PHASE COHERENCE.
To estimate the phase relation of the signals at the different regions, we computed an event related phase coherence analysis (ERPCOH). The ERPCOH is a frequency-domain measure of the synchronization between two channel activities. Its magnitude varies between 0 and 1 where 1 indicates the maximum synchronization. To study the connections between scalp electrodes overlapping the fronto-parietal network, we selected 14 channels of the 183 sites. We decided to use a small number of electrodes to lower the number of comparisons in the statistical procedures. The electrodes selection was in accordance with the extended 10/20 system and covered medially and laterally the frontal, central, and posterior regions of the left and right sides of the scalp. The electrodes selection was not based on the ERSP analysis neither electrode sites were the same as those used in the S-ROIs. In addition, the selection was based on some motor planning and reaching investigations (Bernier et al. 2009; Gerloff et al. 1998). The selected electrodes were: FC3, FC4, C3, C4, CP3, CP4, P3, P4, PO3, PO4, FCZ, CZ, PZ, OZ.
The continuous individual raw EEG signal at selected sites was decomposed with FFT with Hanning tapers. After that, for each channel pair, we computed coherence estimates at any time and frequency as reported by Delorme and Makeig (2004). Mean coherence values for selected time intervals and frequency bands (as in the ERSP analysis) were then determined.
To assess variation of coherence across time, for each frequency band, the mean coherence value was compared between the selected time windows and the baseline (the baseline period was the same as in the ERSP analysis). To determine whether the observed differences were significant, we used a statistical nonparametric procedure. Paired sample t-test were computed between each pair of electrodes and the significant criteria established on the basis of permutation testing (Nichols and Holmes 2002). This procedure accounts for multiple comparisons problem and has been previously applied on EEG signal (Huber et al. 2004).
RESULTS
Visually guided movements are under both feedforward and feedback control
As in previous experiments, the hand paths of the visually guided reaching movements were essentially straight with sharp reversals and out-and-back overlapping strokes (Fig. 1A) as also shown by the values of mean normalized area. The velocity profiles of the outgoing movements were mostly single-peaked and bell-shaped with clear acceleration and deceleration phases. Table 1 reports the means of several kinematic measurements, including reaction times and movement duration. Interestingly, the average amplitudes of peak acceleration and peak velocity were highly and positively correlated (r = 0.96, P < 0.0001), indicating that 92% of the variance in peak velocity (r2 = 0.92) can be explained by variations in peak acceleration alone. However, the variance in movement extent was only partially dependent on the amplitude of peak velocity (r = 0.65, P < 0.02; r2 = 0.42) and peak acceleration (r = 0.43, P > 0.05; r2 = 0.18), an indication that on-line adjustments of the initial movement plan had occurred during the movement itself. Additional support comes also from the finding of a partial dependence of movement extent from movement duration (r = 0.43, P > 0.05; r2 = 0.18). Altogether, these findings suggest that the extent of visually guided reaching movements results from the combination of a preplanned, initial phase—that thus depends on feedforward processes—and by subsequent, on-line adjustments implemented by feedback. In the following paragraphs, we will describe the changes in EEG power occurring during different time windows and their contribution to feedforward and -back processes.
Table 1.
Behavioral results
| Kinematic Measurements | |
|---|---|
| Mean | |
| Reaction time, ms | 233.60 ± 7.67 |
| Movement time, ms | 238.74 ± 7.51 |
| Movement extent, cm | 5.13 ± 0.16 |
| Peak acceleration, cm/s2 | 403.94 ± 19.63 |
| Peak velocity, cm/s | 40.79 ± 1.44 |
| Hand path area | 0.07 ± 0.00 |
| Path curvature (°) | 5.30 ± 0.32 |
| Symmetry index (%) | 3.86 ± 0.24 |
| Variability (SD) | |
| Reaction time, ms | 29.44 ± 1.34 |
| Movement time, ms | 27.32 ± 1.64 |
| Movement extent, cm | 0.67 ± 0.05 |
| Peak acceleration, cm/s2 | 88.66 ± 5.48 |
| Peak velocity, cm/s | 6.94 ± 0.51 |
| Hand path area | 0.08 ± 0.00 |
| Path curvature (°) | 4.51 ± 0.26 |
| Symmetry index (%) | 3.24 ± 0.19 |
Performance indices obtained in the whole group (n = 13).
Temporal changes in EEG power through scalp locations and frequency bands
Figure 2A shows group ERSP plots for each of the five frequency ranges averaged within the five temporal windows that were derived from kinematic measurements (see methods). In general, compared with the baseline, a massive variation in power was evident across all frequency bands and involved electrode sites overlaying the fronto-parietal network that has been associated with motor control.
THETA BAND.
Right after the presentation of the stimuli and before the movement onset, a robust increase of theta power occurred predominantly over the left sensory-motor site that spread ipsilaterally and reached its amplitude peak over the central electrodes during the reaching movement. The theta synchronization over the central sites decreased after the reversal when subjects automatically returned to the center.
ALPHA BAND.
In general, electrode sites over the parietal and parieto-occipital areas showed a desynchronization across the entire epoch with a slight increase of power over the very central sensors. In particular, for the low alpha range (alpha-1), the desynchronization was predominant over the right and left parietal electrodes throughout the reaching and return movement as well as right after its ending. In addition, a slight increase of power occurred over the central electrodes before the onset and during the movement. For the high alpha range (alpha-2), in the planning interval the desynchronization was mainly seen over the left parietal sites and spread to the right parietal and frontal regions and bilaterally over the motor regions during and after the movement execution. This activity reached its peak during the return portion of the movement.
BETA BAND.
In both low and high beta ranges (beta-1 and beta-2, respectively), we observed a large decrease of power starting from stimulus presentation. In fact, in the temporal window between stimulus presentation and movement onset, the left sensory-motor and parietal sensors showed a desynchronization that peaked during the movement and lasted after its ending. In addition, a decrease of power occurred over the medio-frontal and central regions and spread during the movement toward the right sensory-motor sites. The power variation within the beta-2 range was greater than in the beta-1 frequency band.
To better characterize the fronto-parietal network dynamics involved in the planning and execution of our motor task, we modeled nine S-ROIs on the basis of the present ERSP results. Figure 2B displays the selected electrode sites for each S-ROI. The time course and the significant changes (bootstrap statistic) of the group spectral perturbation for each S-ROI and for the selected frequency ranges are summarized in Fig. 2C.
Changes in EEG activity predict motor performance
We then determined the contribution of the different S-ROIs and frequency ranges to the processes involved in the planning and corrective processes of movement extent. We thus used the EEG activity in three temporal windows, i.e., before the stimulus appearance, during planning, and during movement execution, and we correlated it with kinematic indices of performance. The results of correlations are reported in Table 2.
Table 2.
Correlations
| L-CL | R-CL | L-PL | R-PL | L-PO | R-PO | C-Fr | C-Med | A-Fr | |
|---|---|---|---|---|---|---|---|---|---|
| PRE-STIMULUS | |||||||||
| Low beta | |||||||||
| Reaction time mean | 0.72 | 0.64 | 0.55 | 0.59 | 0.58 | 0.70 | 0.67 | 0.68 | 0.81 |
| MOTOR PLAN | |||||||||
| Low alpha | |||||||||
| Movement extent | −0.07 | −0.17 | −0.24 | −0.40 | −0.43 | −0.43 | −0.10 | 0.01 | −0.33 |
| Peak velocity | −0.26 | −0.58 | −0.70 | −0.74 | −0.69 | −0.71 | −0.30 | −0.51 | −0.35 |
| Peak acceleration | −0.28 | −0.60 | −0.67 | −0.67 | −0.56 | −0.58 | −0.28 | −0.53 | −0.28 |
| MOTOR EXECUTION | |||||||||
| Theta | |||||||||
| Movement extent | −0.51 | −0.16 | −0.70 | −0.62 | −0.66 | −0.67 | −0.46 | −0.45 | −0.65 |
| Peak velocity | −0.26 | −0.29 | −0.33 | −0.50 | −0.45 | −0.49 | −0.52 | −0.29 | −0.64 |
| Peak acceleration | −0.07 | −0.24 | −0.05 | −0.29 | −0.19 | −0.24 | −0.35 | −0.11 | −0.44 |
| Low alpha | |||||||||
| Movement extent | −0.40 | −0.23 | −0.52 | −0.45 | −0.59 | −0.63 | −0.41 | −0.40 | −0.63 |
| Peak velocity | −0.46 | −0.36 | −0.59 | −0.60 | −0.58 | −0.60 | −0.53 | −0.48 | −0.51 |
| Peak acceleration | −0.36 | −0.25 | −0.46 | −0.43 | −0.38 | −0.39 | −0.37 | −0.36 | −0.38 |
Correlations between scalp regions of interest brain activity and behavioral indices for selected time windows and frequency bands (baseline: 300 ms after movement offset). Significant correlations (abs(r) > 0.6, p < 0.05) are shown in bold. L- and R-CL, left and right centro-lateral scalp regions; L- and R-PL, left and right posterior lateral scalp regions; L- and R-PO, left and right medial pasieto-occipital scalp regions; C-Fr, centro-frontal region; C-Med, centro-medial region; A-Fr, anterior frontal region.
PRESTIMULUS.
The first time window was 250 ms before the stimulus appearance. During this time, all subjects were waiting for the stimulus to appear. At this point, alertness and attention, which are reflected by reaction time, are crucial to produce timely responses. We found that mean reaction times were selectively predicted by the degree of power variation from the baseline in the beta-1 range in the left sensory-motor and frontal areas, as well as in the centromedial, centroparietal and right parieto-occipital regions. A decrease of power in these regions corresponded to faster reaction times, suggesting that the level of attention, alertness and readiness might be related to activity in the low beta range in the areas involved in sensory-motor and attentional processing.
STIMULUS PROCESSING AND MOTOR PLANNING.
The second time window was 250 ms before the movement onset. As reported in Table 1, mean reaction time was 244.5 ms, ranging from 200.3 to 274.9 ms. Thus this time frame corresponds to movement planning in all the subjects as information about the stimulus position is processed to produce a motor program. As described in the earlier paragraphs, the kinematic variables best reflecting feedforward-type mechanisms are peak acceleration and peak velocity (Ghez and Gordon 1987; Gordon et al. 1994a,b). Movement extent, instead, is the product of both planning and on-line corrections. The results of the correlation between EEG and kinematic data are shown in Table 2. Briefly, we found that amplitude of peak velocity and acceleration, but not movement extent, were significantly predicted by activity in the alpha-1 range bilaterally in the parietal regions. In addition, peak velocity was predicted by alpha-1 activity in the parieto-occipital areas. In general, we found that the lower the activity in these regions, the lower the peak acceleration and velocity, suggesting that parietal regions are involved in movement planning.
MOVEMENT EXECUTION.
Finally, we considered the temporal frame from movement initiation to 250 ms after the movement onset. As reported in Table 1, average movement time was 238.7 ms ranging from 195.8 to 281.7 ms. Thus this time window corresponds to the execution of the reaching portion of the movement, which results from the original motor plan and the adjustments that might be implemented on-line. As discussed in the previous paragraphs, the variable best reflecting all these processes is movement extent. The results of the correlations in Table 2 show that movement extent, but not peak velocity and acceleration, were significantly predicted by activity in the theta and in the alpha-1 ranges in the same regions involved in stimulus processing and motor planning, i.e., the parietal and parieto-occipital regions bilaterally. Interestingly, we also found a significant correlation between the activity in the anterior frontal area and both movement extent and peak velocity, but not with peak acceleration.
Altogether these findings suggest that on-line corrections that are implemented after the occurrence of peak acceleration require the combined work of anterior frontal regions with parieto-occipital as well as sensory and motor areas.
Phase coherence changes across temporal windows
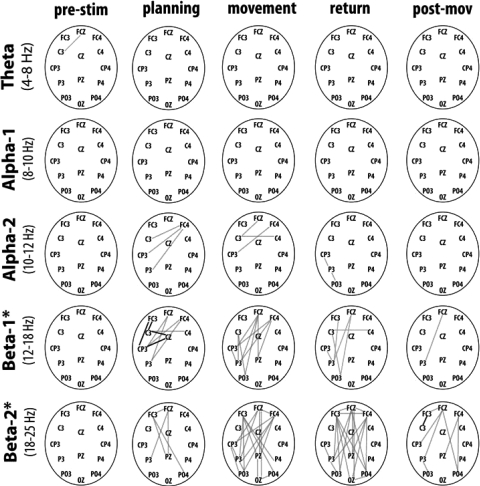
We then evaluated the degree of coherence between the EEG changes at selected sites (see methods for the selection of channels) with ERPCOH. ERPCOH reflects coherent activity between distant areas that are engaged in a specific task. The degree of phase synchronization between two channels, thus, indicates reciprocal interactions between the participating neural populations (Engel et al. 2001). In addition, to determine the variation of coherence as a function of the different stages of reaching, mean coherence estimates within each frequency band at selected channels were compared between the different time intervals of interest and the baseline (nonparametric t-test). The results are reported in Fig. 3. The findings in Fig. 3 and those we summarize below were significant at P < 0.05.
Fig. 3.
Findings obtained in the phase coherence analysis. The different line plotted on the scalp indicates those channel pairs showing significant increase or decrease of coherence values in comparison to the baseline. The coherence variations are plotted for the different time intervals of interest. Gray lines, a significant decrease; black lines, a significant increase (Statistical nonparametric procedure using suprathreshold cluster analysis for multiple comparisons, P < 0.05). Asterisk, for the beta frequency bands, the lines show significant increase/decrease of coherence values in comparison to both the baseline and PreStim time intervals.
BETA COHERENCE IS HIGH BEFORE THE TASK.
Several channel pairs in a diffuse fronto-parietal network showed a significant decrease in phase coherence during the planning and execution of the movement with respect to the baseline and prestimulus periods (Fig. 3). This finding indicates that after the movement offset (baseline period considered in this analysis) and before the presentation of the stimulus, long-range interactions might occur between posterior and anterior sites. For the low beta range, these interactions are mainly contralateral to the movement side, while they occur bilaterally for the high beta band.
In agreement with our findings of significant correlation between beta power variation and reaction times before stimulus presentation, the diffuse early coherent activity in the beta range between the fronto-parietal nodes might reflect general attentional processes that precede target processing and motor planning.
COHERENCE DURING MOVEMENT PLANNING.
In the time window in which motor plan is implemented, channels overlapping the motor and premotor cortices of the contralateral hemisphere showed a selective enhancement of beta coherence. This increase may reflect simultaneous activation and reciprocal connection between cortical regions involved in planning.
DISCUSSION
The results of this study show that when subjects are asked to minimize their reaction time, visually guided movements in a choice-paradigm result from the combination of planning and on-line adjustments that share a similar topographical origin in a fronto-parietal network. However, the two phases differ in terms of the frequency ranges involved. In fact, advanced planning was associated with frequencies in the alpha band; feedback mechanisms instead, mostly involved the alpha-1 and theta band. In addition, we found that in the beta frequency range, phase coherence was always highest before stimulus appearance and decreased during planning and movement execution, and the power significantly predicted reaction times. These are novel findings that suggest that high and low frequencies have differential roles in the modulation of attention, with the former likely reflecting the prestimulus, top-down processes needed to promote timely responses, and the latter being involved in the planning and control of sensorimotor processes.
Visually guided movements in a choice paradigm result from the combination of advanced planning and corrective adjustments
We have found that fast reaching movements in a choice-reaction paradigm incur in trajectory adjustments even without unexpected perturbations: this was evident as extent was not completely predicted by either the amplitude of peak acceleration or peak velocity and adjustments of the initial plan occurred during the movement. The present results are in agreement with those of previous studies on isometric force production (Ghez and Gordon 1987), reaching movements performed without visual guidance in paradigms where subjects moved “when ready” and no minimization of reaction time was requested (Gordon et al. 1994a,b). Interestingly, we have found that peak acceleration and velocity were significantly predicted by low alpha range activity bilaterally in the parietal and parieto-occipital sites in the temporal window corresponding to movement planning. Conversely, movement extent was significantly predicted by theta and low alpha activity recorded over the anterior frontal areas and in parietal and parieto-occipital regions bilaterally in the temporal window corresponding to the movement execution. The specificity of the temporal pattern of these correlations allows us to infer that planning of the force needed to propel the arm toward the target—that is expressed by peak acceleration and velocity—is under the control of parietal-occipital regions, while later adjustments require the activity of the frontal areas that have a general supervisory function. Notably, both planning and supervisory processes employ low frequency ranges. The functional significance of such patterns is discussed in the following paragraphs.
Topography of feedforward and feedback mechanism: overlapping networks
Our results show that in a choice-reaction time paradigm the planning and execution of reaching movements share spatial patterns of activation. As in previous EEG studies on movement (Neuper and Pfurtscheller 2001; Stancak and Pfurtscheller 1995), we found that induced-oscillatory attenuation of alpha and beta rhythms began in the planning stage and lasted till the end of the movement. The patterns of power attenuation might be functionally interpreted as the blocking of coherent activity over large neural pools and thus reflecting activation of the underlying networks. In particular, before movement onset, changes of power were found over the contralateral sensorimotor and parietal sites as well as over the right parietal electrodes (alpha band) and the medio-frontal electrodes (beta band). During movement execution, both frequencies showed the same topographical pattern as in the planning phase with a stronger attenuation of power and with additional involvement of the right sensorimotor regions. Theta frequency band has been seldom investigated in motor studies, and there is only one report of its involvement in the planning of catching in a repetitive predictable task (Tombini et al. 2009). In the present study, we have found that theta is significantly involved not only in the planning but also in the execution of reaching movements. In fact, during movement planning, we observed an increase of theta power over the left sensorimotor areas, which further involved the centro-frontal sites during the movement execution. These results are in line with those of the other EEG investigations with motor tasks (Neuper and Pfurtscheller 2001), although they slightly differ in two respects: we did not observe any beta synchronization after movement-offset and alpha desynchronization was of a smaller magnitude. These two differences might have a common source, namely, our selection of the baseline, that is, the 300 ms period starting ∼250 ms after the movement end in which subjects were completely still. As enhancement of beta power usually occurs in this time window, our baseline selection might have zeroed the beta “burst” and amplified the desynchronization patterns in the planning and execution stages. On the other hand, alpha desynchronization usually lasts for >500 ms after the movement end (Stancak and Pfurtscheller 1996): because this period overlaps with our baseline, we might have seen an attenuation of the alpha ERSP. However, when we performed the very same analysis using the entire epoch as baseline (see supplementary material), we obtained patterns of activations of beta and alpha rhythms that were similar to the ones we found with a smaller baseline in terms of their locations. In addition, we observed a synchronization of alpha and beta after the end of the movement (alpha and beta rebound). Obviously, in this analysis, the amplitude of power variation was attenuated.
With the “naturalistic” paradigm we chose, in which many processes occur in parallel, the simple analysis of EEG oscillatory activity could not provide insights in the specific topography of feedforward and -back mechanisms. Our approach has provided, for the first time, the possibility of capturing and comparing with a single task the temporal unfolding of the many brain processes (attentional, planning and execution) involved in reaching. With the experimental paradigms used in all the previous studies, only one or, at best a few of such processes were isolated and defined. Such break down might have given only a limited, “out of context” view of the processes that did not reflect real life. Therefore we correlated behavioral indices of such mechanisms with regional EEG activity in three temporal windows, and for specific frequency bands. We found that advanced planning indices were significantly predicted by bilateral activity in the S-ROIs over the parietal and parieto-occipital regions in the alpha-1 range in the temporal window corresponding to movement planning. Kinematic indices of corrective mechanisms, instead, were significantly predicted by activity in the anterior frontal S-ROI, in addition to the bilateral involvement of the parietal and parieto-occipital S-ROIs. These correlations were seen in the alpha-1 and theta ranges in the temporal window corresponding to movement execution (of note, the dissociation of correlations between low frequency activity and feedback and feedforward indices in the two temporal windows was also evident in the entire epoch baseline analysis; see supplemental material).
The partial overlapping of the scalp sites involved in feedback and -forward processes, that is, the parietal and parieto-occipital electrodes, is in line with other studies in behaving monkeys (Sabes 2000). Indeed the oscillatory activity observed over the posterior sites and its correlation with feedforward and -back indices might reflect visuo-spatial processing and integration of sensory information that are needed to both motor planning and on-line adjustments. In this regard, the parietal and parieto-occipital cortices are specialized nodes for multisensory integration (Sabes 2000). This seems to be especially true for aimed movements, as shown by reaching experiments carried on in both monkeys (Andersen and Buneo 2002; Batista et al. 1999) and humans (Labyt et al. 2003). The involvement of the frontal activity in the corrective phases of the movement does not come as a surprise. In fact, mid-frontal activity is often responsible for the monitoring and correction of an ongoing performance as shown in investigations on working memory with cognitive paradigms (Gevins et al. 1997). Our results clearly extend the supervising role of frontal area to the reaching behavior, as suggested by the results of previous studies (Contreras-Vidal and Kerick 2004; Tombini et al. 2009).
High and low rhythms differentially modulate attentional processes
Converging evidence in our study suggests that high and low brain rhythms might have differential roles in the modulation of the attentive processes needed for movement planning and low execution: high-frequency range is likely more involved in top-down processes promoting prompt responses; low-frequency range in planning and controlling functions.
In fact, in agreement with previous studies (Hanslmayr et al. 2007; Zhang et al. 2008), we found a significant correlation between beta power variation and reaction times. Plus before stimulus appearance, long-range phase coupling between anterior and posterior electrodes in the beta frequency occurred, as revealed by the significant decrease during planning an execution of the movement (Fig. 3). Therefore this frequency might play a pivotal role in the modulation of top-down attentional processes that are likely needed to enhance perception and to promote prompt responses to task-relevant stimuli.
It is possible that the high beta coherence seen before stimulus appearance reflects the increased oscillatory activity following movement offset. However, our data suggest that the prestimulus beta coherence is the expression, at least in part, of the formation of transient neural assemblies responsible for efficient perception and motor performance through the modulation of attention (see supplemental material). Indeed the baseline we selected, that is, the period preceding stimulus process and motor execution, allowed us to capture the fast power and coherence variation associated with the disengagement from the beta and alpha “idle” or “active inhibition” state that follows the movement (Pfurtscheller 2003). This aspect is particularly crucial to interpret the positive correlation between beta-1 attenuation over posterior and anterior sites and reaction times: the faster is the disengagement from the idle state, the faster are the stimulus processing and movement planning. This brain dynamics would have been obscured by the selection of the entire epoch as reference (see supplemental material).
Our conclusions on the role of beta activity are in agreement with the results of two recent studies showing a strong correlation between performance in a visual perceptual task and beta phase synchrony in humans (Hanslmayr et al. 2007; Zhang et al. 2008) and between reaction time and beta power in monkeys performing a visuomotor discrimination task (Zhang et al. 2008).
Theta synchronization, instead, peaked before and during the outgoing part of the movement and decreased when on-line feedback was not needed anymore. In addition, theta and alpha-1 activity in parietal and frontal sites was significantly correlated with an index of feedback control in the temporal window corresponding to movement execution. Therefore low frequency band is likely involved in the attentional mechanisms that are specifically required for the planning and the on-line adjustments of the movement. Attentional and working memory resources are needed during the planning of target-directed movements and midline frontal activation, which is fundamental for allocation of cognitive resources (Sauseng et al. 2007) and for supervising the performance (Rodriguez-Fornells et al. 2002), might be required in the development of motor plans as well as in error detection and correction. These conclusions are supported by the results of many cognitive studies demonstrating that theta activity reflects the amount of attentional resources and effort engaged in a specific task (Gevins et al. 1997; Gomarus et al. 2006). In addition, one recent motor study reports that motor-related enhancement of theta activity occurred in frontal and sensorimotor areas in the planning stage of reaching and catching (Tombini et al. 2009). Interestingly, these authors also observed decreased frontal theta activation as a function of practice and improvement, even if some others have found an increase of theta frontal power (Gentili et al. 2010). The direction of the changes in theta power might likely depend on the nature of the task under investigation as well as on the considered theta band component (high or low). Altogether, these and our results suggest that theta oscillations might play a fundamental role in attention and sensorimotor integration processes as well as in feedback mechanisms.
GRANTS
This work was supported by grants from the McDonnell Foundation to G. Tononi and M. F. Ghilardi, by grants from National Parkinson Foundation, Rudin Fellowship and American Parkinson's Association to M. F. Ghilardi, and National Institute of Health Grants NS-054864 to M. F. Ghilardi, NS-055185 to G. Tononi, and P20MH-077967 to G. Tononi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The first author thanks F. Mons for the kind and precious support to the study.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220, 2002 [DOI] [PubMed] [Google Scholar]
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science 285: 257–260, 1999 [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M. Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex 13: 1009–1022, 2003 [DOI] [PubMed] [Google Scholar]
- Bernier PM, Burle B, Hasbroucq T, Blouin J. Spatio-temporal dynamics of reach-related neural activity for visual and somatosensory targets. Neuroimage 47: 1767–1777, 2009 [DOI] [PubMed] [Google Scholar]
- Bradberry TJ, Gentili RJ, Contreras-Vidal JL. Reconstructing three-dimensional hand movements from noninvasive electroencephalographic signals. J Neurosci 30: 3432–3437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Kerick SE. Independent component analysis of dynamic brain responses during visuomotor adaptation. Neuroimage 21: 936–945, 2004 [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol 16: 205–212, 2006 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004 [DOI] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci 2: 563–567, 1999 [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Principle component analysis of ERP data. In: Event-Related Potentials A Methods Handbook, edited by Handy TC. Cambridge, MA: MIT Press, p. 189–207, 2005 [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2: 704–716, 2001 [DOI] [PubMed] [Google Scholar]
- Gentili RJ, Bradberry TJ, Oh H, Hatfiled BD, Contreras-Vidal JL. Cerebral cortical dynamics during visuomotor transformation: Adaptation to a cognitive-motor executive challenge. Psychophysiology (October 21, 2010). doi:10.1111//j.1469-8986.2010.01143.x [DOI] [PubMed] [Google Scholar]
- Gerloff C, Richard J, Hadley J, Schulman AE, Honda M, Hallett M. Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121: 1513–1531, 1998 [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex 7: 374–385, 1997 [DOI] [PubMed] [Google Scholar]
- Ghez C, Gordon J. Trajectory control in targeted force impulses. I. Role of opposing muscles. Exp Brain Res 67: 225–240, 1987 [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F. Visual feedback has differential effects on reaching movements in Parkinson's and Alzheimer's disease. Brain Res 876: 112–123, 2000 [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology 60: 1313–1319, 2003 [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW. Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J Neurophysiol 101: 2218–2229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomarus HK, Althaus M, Wijers AA, Minderaa RB. The effects of memory load and stimulus relevance on the EEG during a visual selective memory search task: an ERP and ERD/ERS study. Clin Neurophysiol 117: 871–884, 2006 [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Cooper SE, Ghez C. Accuracy of planar reaching movements. II. Systematic extent errors resulting from inertial anisotropy. Exp Brain Res 99: 112–130, 1994a [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Accuracy of planar reaching movements. I. Independence of direction and extent variability. Exp Brain Res 99: 97–111, 1994b [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Woods RP, Arbib MA. Functional anatomy of pointing and grasping in humans. Cereb Cortex 6: 226–237, 1996 [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bauml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage 37: 1465–1473, 2007 [DOI] [PubMed] [Google Scholar]
- Higgins JR, Angel RW. Correction of tracking errors without sensory feedback. J Exp Psychol 84: 412–416, 1970 [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Functional specialization in dorsal and ventral premotor areas. Prog Brain Res 143: 507–511, 2004 [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature 430: 78–81, 2004 [DOI] [PubMed] [Google Scholar]
- Labyt E, Szurhaj W, Bourriez JL, Cassim F, Defebvre L, Destee A, Guieu JD, Derambure P. Changes in oscillatory cortical activity related to a visuomotor task in young and elderly healthy subjects. Clin Neurophysiol 114: 1153–1166, 2003 [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Kramer GF, Jensen O, Oostenveld R, Schoffelen JM, Fries P. Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb Cortex 17: 2364–2374, 2007 [DOI] [PubMed] [Google Scholar]
- Moisello C, Bove M, Huber R, Abbruzzese G, Battaglia F, Tononi G, Ghilardi MF. Short-term limb immobilization affects motor performance. J Mot Behav 40: 165–176, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisello C, Crupi D, Tunik E, Quartarone A, Bove M, Tononi G, Ghilardi MF. The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task. Exp Brain Res 194: 143–155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo JR, Brovelli A, Longo R, Budai R, Kristeva R, Battaglini PP. EEG dynamics of the frontoparietal network during reaching preparation in humans. Neuroimage 34: 1673–1682, 2007 [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol 43: 41–58, 2001 [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Induced oscillations in the alpha band: functional meaning. Epilepsia 44, Suppl 12: 2–8, 2003 [DOI] [PubMed] [Google Scholar]
- Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y. An “automatic pilot” for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 3: 729–736, 2000 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Kurzbuch AR, Munte TF. Time course of error detection and correction in humans: neurophysiological evidence. J Neurosci 22: 9990–9996, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabes PN. The planning and control of reaching movements. Curr Opin Neurobiol 10: 740–746, 2000 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur J Neurosci 25: 587–593, 2007 [DOI] [PubMed] [Google Scholar]
- Senot P, Baillet S, Renault B, Berthoz A. Cortical dynamics of anticipatory mechanisms in interception: a neuromagnetic study. J Cogn Neurosci 20: 1827–1838, 2008 [DOI] [PubMed] [Google Scholar]
- Stancak A, Jr, Pfurtscheller G. Desynchronization and recovery of beta rhythms during brisk and slow self-paced finger movements in man. Neurosci Lett 196: 21–24, 1995 [DOI] [PubMed] [Google Scholar]
- Stancak A, Jr, Pfurtscheller G. Mu-rhythm changes in brisk and slow self-paced finger movements. Neuroreport 7: 1161–1164, 1996 [DOI] [PubMed] [Google Scholar]
- Tombini M, Zappasodi F, Zollo L, Pellegrino G, Cavallo G, Tecchio F, Guglielmelli E, Rossini PM. Brain activity preceding a 2D manual catching task. Neuroimage 47: 1735–1746, 2009 [DOI] [PubMed] [Google Scholar]
- Van Der Werf J, Jensen O, Fries P, Medendorp WP. Neuronal synchronization in human posterior parietal cortex during reach planning. J Neurosci 30: 1402–1412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario DS, Ghez C. The control of rapid limb movement in the cat. IV. Updating of ongoing isometric responses. Exp Brain Res 55: 134–144, 1984 [DOI] [PubMed] [Google Scholar]
- Virji-Babul N, Moiseev A, Cheung T, Weeks D, Cheyne D, Ribary U. Spatial-temporal dynamics of cortical activity underlying reaching and grasping. Hum Brain Mapp 31: 160–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth RS. The accuracy of voluntary movement. Psych Rev 3: 1–106, 1899 [Google Scholar]
- Zhang Y, Wang X, Bressler SL, Chen Y, Ding M. Prestimulus cortical activity is correlated with speed of visuomotor processing. J Cogn Neurosci 20: 1915–1925, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.