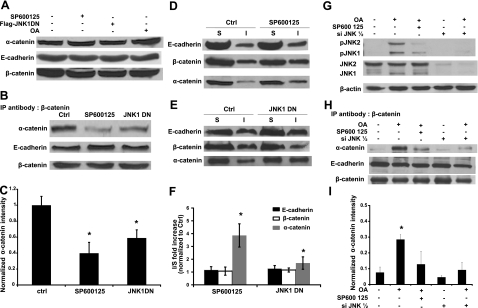
Figure 3.
JNK kinase activity is necessary for the association of α-catenin with β-catenin and E-cadherin. Human primary keratinocytes remained untreated (ctrl), they were treated with SP600125 (SP600125, 10 μM), they were genetically modified to express dominant negative JNK1, or they were treated with okadaic acid (OA, 500 nM). A) Cell lysates were subjected to Western blotting with antibody against α-catenin. E-cadherin and β-catenin were used as loading control. B) Cell lysates were immunoprecipitated with an antibody against β-catenin and subsequently subjected to Western blotting for α-catenin and E-cadherin. β-Catenin was used as loading control. C) Lane intensity of α-catenin was analyzed by Kodak gel documentation software and normalized to β-catenin. D, E) After Triton-X100 extraction, soluble (S) and insoluble (I) fractions were separated and subjected to Western blotting for E-cadherin, β-catenin, and α-catenin. F) For each of the indicated proteins, the ratio of the band intensity of the Triton X-100 insoluble over the soluble fractions after JNK inhibition (SP600125 or JNK1DN) was normalized to the same ratio of control cells. G) A431 or siJNK1/2 A431 cells were treated with okadaic acid (OA, 500 nM) for 30 min in the presence or absence SP600125 (10 μM). Cell lysates were subjected to Western blotting for phosphor JNK and total JNK; β-actin served as a loading control. H) Cells were treated as in G, and cell lysates were immunoprecipitated with antibody against β-catenin and subsequently subjected to Western blotting for α-catenin and E-cadherin. β-Catenin was used as loading control. I) Lane intensity of α-catenin was analyzed by Kodak gel documentation software and normalized to β-catenin. *P < 0.05 vs. control.