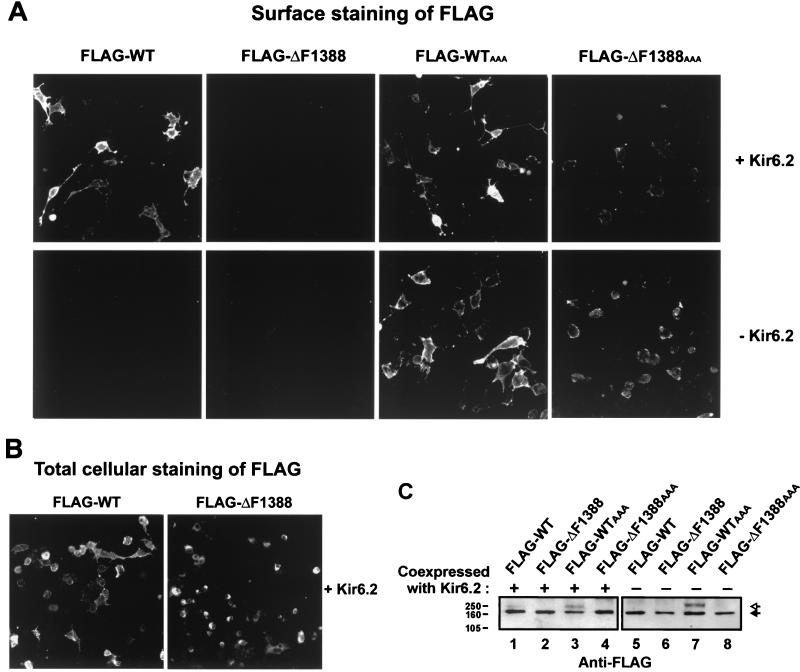
Figure 2.
Lack of surface expression of ΔF1388 SUR1 mutant KATP channels. (A) COSm6 cells transiently transfected with FLAG-wild type (WT) SUR1, FLAG-ΔF1388 SUR1, or FLAG-ΔF1388 SUR1AAA, in the presence (Upper) or absence (Lower) of Kir6.2, were immunostained for the FLAG epitope. When coexpressed with Kir6.2, surface staining was clearly observed with FLAG-WT SUR1 but not with FLAG-ΔF1388 SUR1. Mutating the RKR sequence to AAA in SUR1 (FLAG-ΔF1388AAA SUR1) partially overcomes the trafficking defect caused by the ΔF1388 mutation and allows surface expression of the mutant protein both in the presence (Upper) and in the absence (Lower) of Kir6.2. (B) COSm6 cells transiently expressing Kir6.2 and FLAG-WT SUR1 or FLAG-ΔF1388 SUR1 were fixed with methanol and immunostained for the FLAG epitope. The FLAG-WT SUR1 and the FLAG-ΔF1388 SUR1 showed equivalent levels of staining, suggesting the wild type and the mutant protein are expressed at similar levels. A strong perinuclear staining pattern indicative of ER accumulation was observed with FLAG-ΔF1388 SUR1, whereas less perinuclear and more membrane staining was seen with FLAG-WT SUR1. (C) FLAG-WT, FLAG-ΔF1388, FLAG-WTAAA, and FLAG-ΔF1388AAA SUR1 constructs were expressed in the presence or absence of Kir6.2 and detected by immunoblotting with antibody to the FLAG tag. Solid arrow indicates core glycosylated SUR1; open arrow denotes complex glycosylated SUR1 seen with FLAG-WT coexpressed with Kir6.2 (lane 1) and FLAG-WTAAA (lanes 3 and 7). Molecular mass markers (kDa) are indicated at left. Note that the amount of DNA used for transfection in the two blots were different (see Materials and Methods), so intensities of bands cannot be compared between blots.