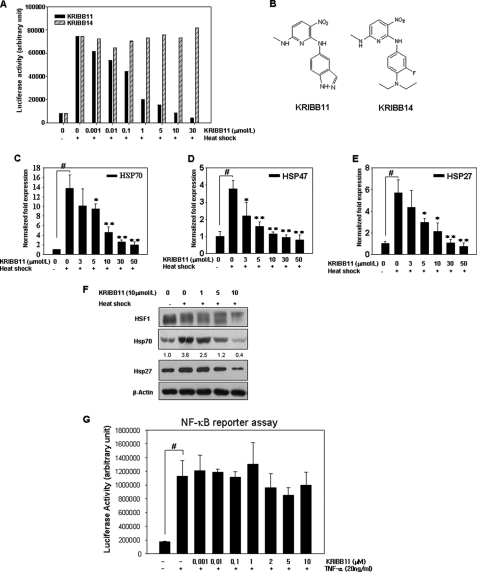
FIGURE 1.
KRIBB11 inhibits HSF1 activity in a concentration-dependent manner. A, HCT-116 cells were transfected with the p(HSE)4-TA-Luc reporter plasmid and treated with or without heat in the presence of different concentrations (0.001–30 μmol/liter) of KRIBB11 or KRIBB14. Reporter assay was carried out as described under “Experimental Procedures.” B, structure of KRIBB11 and KRIBB14. C–E, KRIBB11 inhibited heat shock-induced transcription of hsp70, hsp47, and hsp27 in a concentration-dependent manner. HCT-116 cells were treated with KRIBB11 (3–50 μmol/liter) for 30 min at 37 °C, exposed to heat shock at 43 °C for 1 h, and then incubated further at 37 °C for 1 h; quantitative analysis of mRNA levels of hsp70, hsp47, and hsp27 was carried out using real time PCR. The expression of each mRNA was normalized against the GAPDH gene. Each experiment was repeated three times, and each value is a mean ± S.D. Statistical significance (p value) was determined with an unpaired t test. #, p < 0.01 versus no heat control. *, p < 0.05; **, p < 0.01 versus heat control. F, KRIBB11 inhibited heat-induced HSP70 and HSP27 expression in HCT-116 cells. HCT-116 cells were treated with the indicated concentrations of KRIBB11 for 30 min, exposed to heat shock at 43 °C for 1 h, and then incubated further at 37 °C for 5 h. Whole cell lysates were analyzed by Western blotting as described under “Experimental Procedures.” G, HeLa cells expressing the pNF-κB-Luc reporter were pretreated for 30 min with the indicated concentrations of KRIBB11 and then stimulated for 16 h with TNF-α (20 ng/ml). Luciferase activity was determined with a GloMaxTM 96 microplate luminometer (Promega). Each experiment was repeated two times, and each value is the mean ± S.D. Statistical significance (p value) was determined with an unpaired t test. #, p < 0.01 versus control.