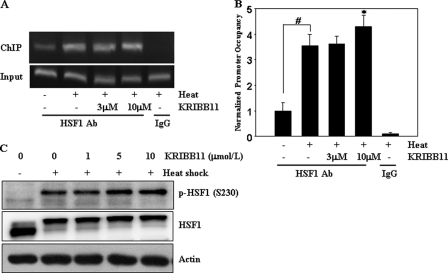
FIGURE 4.
KRIBB11 did not inhibit heat shock-induced HSF1 binding to the hsp70 promoter or phosphorylation of HSF1 Ser-230. A and B, ChIP analysis of HSF1 binding to the hsp70 promoter. HCT-116 cells were incubated with the indicated concentrations of KRIBB11, heat-shocked at 43 °C for 1 h, and analyzed for recruitment of HSF1 to the hsp70 promoter by the ChIP assay as described under “Experimental Procedures.” ChIP-enriched DNAs using preimmune IgG or anti-HSF1 antibody, as well as input DNAs, were prepared. Quantification of the DNA fragment of the hsp70 gene (−216 to −24) was done using PCR (A) or real time PCR (B). The ChIP assay was done three times, and similar results were obtained. Relative promoter occupancy is expressed as fold induction compared with the control prepared from samples that were not heat treated. Error bars indicate ± S.D. Statistical significance (p value) was determined with an unpaired t test. #, p < 0.01 versus no heat control. *, p < 0.05 versus heat control. C, phosphorylation of HSF1 on serine 230. HCT-116 cells were incubated with the indicated concentrations of KRIBB11, heat-shocked at 43 °C for 1 h, and then incubated at 37 °C for 5 h. HCT-116 cell lysates were analyzed by immunoblotting using an anti-phospho-HSF1 Ser-230 antibody as described under “Experimental Procedures.”