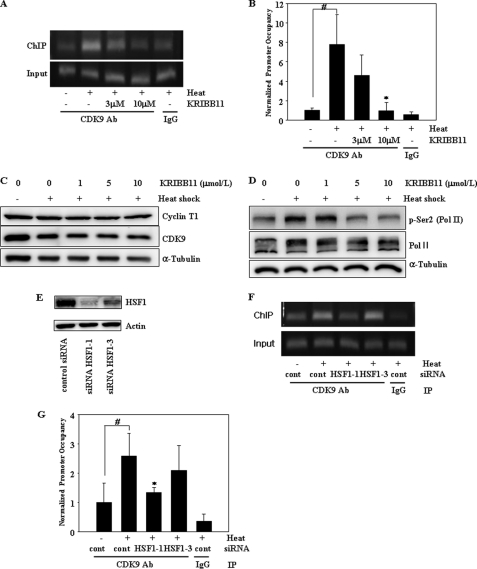
FIGURE 5.
KRIBB11 inhibits heat shock-induced recruitment of p-TEFb to the hsp70 promoter and phosphorylation of RNA pol II CTD Ser-2. A and B, ChIP analysis of CDK9 of the p-TEFb subunit. HCT-116 cells were incubated with the indicated concentrations of KRIBB11, heat-shocked at 43 °C for 1 h, and then analyzed for the recruitment of p-TEFb to the hsp70 promoter by ChIP assay as described under “Experimental Procedures.” ChIP-enriched DNAs using preimmune IgG or anti-CDK9 antibody, as well as input DNAs, were prepared. Quantification of the DNA fragment of the hsp70 gene (−26 to +115) was done using PCR (A) or real time PCR (B). The ChIP assay was repeated three times. Relative promoter occupancy is expressed as fold induction compared with the control prepared without heat shock. Error bars indicate ± S.D. Statistical significance (p value) was determined with an unpaired t test. #, p < 0.01 versus no heat control. *, p < 0.05 versus heat control. C, expression of cyclin T1 and CDK9. HCT-116 cells were incubated with the indicated concentrations of KRIBB11, heat-shocked at 43 °C for 1 h, and then incubated at 37 °C for 5 h. Equal amounts of lysates were analyzed by Western blotting using anti-cyclin T1 or anti-CDK9 antibodies as described under “Experimental Procedures.” D, phosphorylation of RNA pol II CTD Ser-2. HCT-116 cells were incubated with the indicated concentrations of KRIBB11 and heat-shocked at 43 °C for 1 h, and then incubated at 37 °C for 5 h. HCT-116 lysates were analyzed by Western blotting using anti-phospho-Ser-2 or RNA pol II antibody as described under “Experimental Procedures.” E, knockdown of HSF1 by siRNA. HCT-116 cells were transfected with the indicated siRNAs for 72 h. siRNA-transfected cells were lysed, and equal amounts of protein were subjected to immunoblotting with the indicated antibodies. F and G, ChIP analysis of CDK9 of the p-TEFb subunit. HCT-116 cells were transfected with the indicated siRNAs for 72 h, heat-shocked at 43 °C for 1 h, and then analyzed for recruitment of p-TEFb to the hsp70 promoter by ChIP assay as described under “Experimental Procedures.” ChIP-enriched DNAs using preimmune IgG or anti-CDK9 antibody, as well as input DNAs, were prepared. Quantification of the DNA fragment of the hsp70 gene (−26 to +115) was done using PCR (F) or real time PCR (G). Statistical significance (p value) was determined with an unpaired t test. #, p < 0.05 versus no heat control. *, p < 0.05 versus heat control.