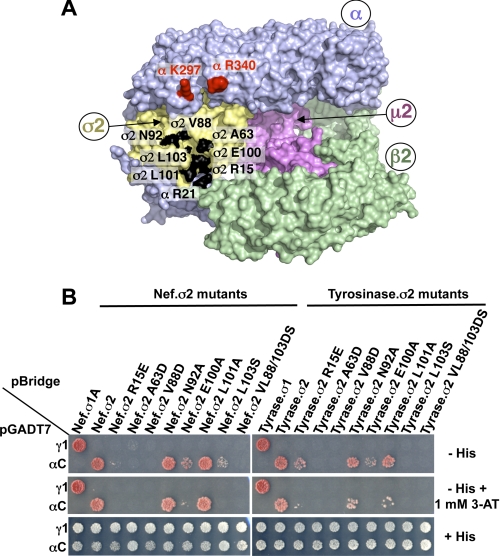
FIGURE 3.
Analysis of AP-2 residues involved in the interaction with Nef and tyrosinase (D/E)XXXL(L/I) signals. A, residues in the α (Arg21) and σ2 (Ala63, Val88, Asn92, Glu100, Leu101, and Leu103) subunits that were subjected to mutagenesis are shown in black on the surface representation of the three-dimensional structure of the AP-2 core complex (Protein Data Bank codes 1GW5 and 2VGL) (50). The α, β2, μ2, and σ2 subunits are depicted in light blue, green, magenta, and gold, respectively. Shown in red on the α subunit are residues Lys297 and Arg340, which are also required for the binding of the AP-2 α-σ2 hemicomplex to HIV-1 Nef (27). B, Y3H assays showing the effect of σ2 substitutions on the interaction of the AP-2 α-σ2 hemicomplex with HIV-1 Nef and tyrosinase (Tyrase) (D/E)XXXL(L/I) signals. Experiments were performed as indicated in the legend to Fig. 2. Positive controls included the interaction of (D/E)XXXL(L/I) signals with the AP-1 γ-σ1 and AP-2 α-σ2 hemicomplexes, whereas double transformants expressing (D/E)XXXL(L/I) signals and discordant γ and σ2 pairs were used as negative controls. Double transformants were plated on −His and −His plus 1 mm 3-AT medium to analyze the interactions at different levels of stringency and on +His medium as a control for loading and viability. The image shown represents a composite of different plates from the same experiment.