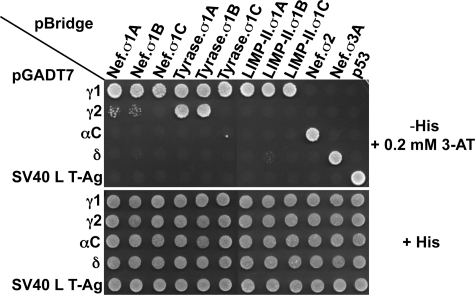
FIGURE 9.
γ subunit- and motif-dependent interaction of AP-1 with (D/E)XXXL(L/I) signals. All γ1-based AP-1 hemicomplexes (γ1-σ1A, γ1-σ1B, and γ1-σ1C) display similarly high avidities for (D/E)XXXL(L/I) signals. In contrast, γ2-σ1A and γ2-σ1B interact weakly with HIV-1 Nef, interact strongly with the tyrosinase tail, and do not recognize the LIMP-II signal. The lack of interaction detected for all double transformants expressing γ2 and σ1C is consistent with lack of assembly of AP-1 complexes comprising these two subunits, as evidenced by immunoprecipitation-recapture analysis of metabolically labeled cells (Fig. 8). This lack of interaction cannot be explained by lack of expression of γ2 and σ1C subunits in yeast given the binding of the tyrosinase signal to γ2-σ1A or γ2-σ1B and to γ1-σ1C, respectively. The image shown represents a composite of different plates from the same experiment. SV40 L T-Ag, SV40 large T-antigen (negative control for interactions with pBridge constructs and positive control for interaction with p53).