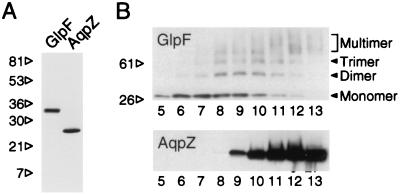
Figure 1.
SDS/PAGE and velocity sedimentation analyses of His-GlpF and His-AqpZ. (A) Protein samples (≈1 μg) in 1% SDS and 140 mM 2-mercaptoethanol were analyzed with 10–20% acrylamide gradient gel slabs and stained with Coomassie brilliant blue (34). AqpZ samples were previously acidified with HCl and neutralized to disrupt the SDS-stable tetramer (1). (B) Membranes from transformed bacteria were solubilized in OG, layered over a 5–20% continuous sucrose gradient, and sedimented at 140,000 × g for 18 hr at 4°C. Twenty fractions were collected; top of gradient is on the left. Mobility of His-GlpF or His-AqpZ was determined by immunoblotting.