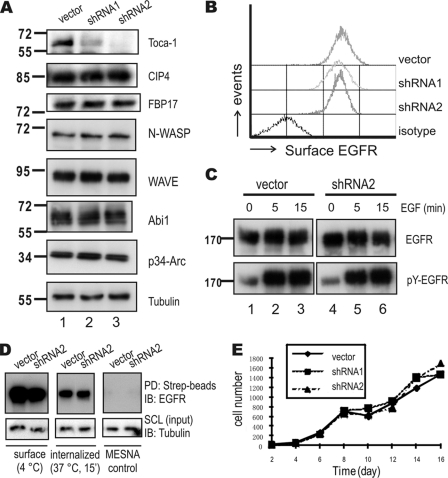
FIGURE 3.
Toca-1 knockdown in A431 cells does not affect EGFR internalization or cell growth. A, A431 cells transduced with vector control or two Toca-1 shRNA-expressing lentiviruses (shRNA1 and shRNA2) were selected with puromycin. After several passages, cell lysates were prepared and subjected to IB with antiserum specific for Toca-1, CIP4, FBP17, N-WASP, WAVE, Abi1, p34-Arc, and tubulin. Positions of molecular mass markers are shown on the left. B, surface EGFR levels were compared between cell lines by staining with R-phycoerythrin-conjugated anti-EGFR and isotype control followed by flow cytometry. C, A431 vector control and Toca-1 knockdown (shRNA2) cells were serum-starved and treated with or without EGF (100 ng/ml) for 5 or 15 min. Soluble cell lysates were subjected to IB with antiserum specific for active-EGFR (pY-EGFR) and EGFR. Panels for vector and Toca-1 knockdown cells are taken from the same autoradiographs of equal exposure time. D, EGFR endocytosis assays for A431 vector and Toca-1 knockdown (shRNA2) were carried out using surface biotinylation, as described under “Experimental Procedures.” Following biotinylation, cells were either kept at 4 °C (surface) or subjected to quenching with MESNA following incubation at 37 °C for 15 min (internalized). As a negative control, samples were quenched immediately after biotinylation (MESNA control). SCL were subjected to IB with tubulin (input loading control) or pull-down (PD) using streptavidin-Sepharose (Strep-beads) followed by IB with mouse anti-EGFR. E, the graph depicts average cell counts for vector control (solid line) and Toca1 knockdown cells (dashed lines) between 2 and 16 days of plating.