Abstract
Polar auxin movement is a primary regulator of programmed and plastic plant development. Auxin transport is highly regulated at the cellular level and is mediated by coordinated transport activity of plasma membrane-localized PIN, ABCB, and AUX1/LAX transporters. The activity of these transporters has been extensively analyzed using a combination of pharmacological inhibitors, synthetic auxins, and knock-out mutants in Arabidopsis. However, efforts to analyze auxin-dependent growth in other species that are less tractable to genetic manipulation require more selective inhibitors than are currently available. In this report, we characterize the inhibitory activity of 5-alkoxy derivatives of indole 3-acetic acid and 7-alkoxy derivatives of naphthalene 1-acetic acid, finding that the hexyloxy and benzyloxy derivatives act as potent inhibitors of auxin action in plants. These alkoxy-auxin analogs inhibit polar auxin transport and tropic responses associated with asymmetric auxin distribution in Arabidopsis and maize. The alkoxy-auxin analogs inhibit auxin transport mediated by AUX1, PIN, and ABCB proteins expressed in yeast. However, these analogs did not inhibit or activate SCFTIR1 auxin signaling and had no effect on the subcellular trafficking of PIN proteins. Together these results indicate that alkoxy-auxins are inactive auxin analogs for auxin signaling, but are recognized by PIN, ABCB, and AUX1 auxin transport proteins. Alkoxy-auxins are powerful new tools for analyses of auxin-dependent development.
Keywords: ABC Transporter, Chemical Modification, Hormones, Medicinal Chemistry, Plant, Auxin Transport, Inhibitor, Plant Hormone
Introduction
The plant hormone auxin plays an essential role in embryogenesis, cell elongation, vascular tissue differentiation, tropic responses to light and gravity, and lateral branching of shoots and roots (1). Auxin levels are regulated by biosynthesis, polar transport, and the catabolism of the signal molecule. Polar auxin streams function in the establishment and maintenance of the embryonic apical-basal axis, organogenesis, lateral development, root extension, and vascular patterning (2–6). Asymmetric redistribution of auxin in response to light, gravity, and touch stimuli establishes gradients that direct tropic growth that allows plants to adapt to environmental inputs and maintain positioning in relation to the gravity vector and light (7–10). Molecular genetic studies in the model system Arabidopsis thaliana and other species have shown that a combination of transport systems comprising the PINFORMED (PIN) efflux carriers, ATP-binding cassette group B (ABCB) auxin transporters, and AUX1/LAX uptake permeases coordinately mobilize auxin transport streams (9, 11). The AUX1 influx transporter is a transmembrane protein similar to amino acid permeases. AUX1 mediates apolar uptake of auxin in lateral root cap cells to motivate redirection of shoot-derived vascular auxin streams, and loss of AUX1 function results in agravitropic root growth. However, AUX1 does not appear to directly mediate lateral redirection of auxin streams in gravitropic bending, as gravitropic growth can be restored by treatment of Arabidopsis aux1–7 mutants with the membrane-permeable artificial auxin naphthalene 1-acetic acid (NAA)3 (12). AUX1 transports the primary natural auxin indole-3-acetic acid (IAA) and 2,4-dichlorophenoxy acetic acid (2,4-D: a synthetic auxin), but not NAA (13, 14). PIN proteins are auxin efflux facilitators polarly localized on the plasma membrane that mediate polar auxin transport required for organogenesis and tropic growth. ABCB1 and ABCB19 auxin efflux transporters primarily function in apolar auxin efflux and motivate loading of auxin into polar streams and maintenance of long distance auxin transport (9). Another member of the family, ABCB4, has been shown to mediate both auxin influx and efflux transport depending on auxin concentration, the presence of transport inhibitors, and other cellular factors (14).
Much of what is known about polar auxin transport is derived from studies using pharmacological inhibitors. Both PIN and ABCB auxin efflux activities are noncompetitively inhibited by treatment with 1-naphthylphthalamic acid (NPA), 1-(2′-carboxyphenyl)-3-phenylpropane-1,3-dione, and pyrenoyl benzoic acid (PBA) (15–18). However, NPA also inhibits interactions between PIN and ABCB proteins (18) and ABCB interactions with the co-chaperone FKBP42 (19, 20). At higher concentrations, NPA also inhibits the M1 aminopeptidase APM1 (21). 1-Naphthoxyacetic acid has been shown to inhibit auxin uptake mediated by AUX1/LAX proteins (22, 23). 2,3,5-Triiodobenzoic acid (TIBA) has also been used extensively as an auxin transport inhibitor, but also has weak auxin activity itself (24). All of these inhibitors nonspecifically inhibit other cellular processes at higher concentrations (25, 26).
Concerns about a lack of specificity in these “classical” auxin transport inhibitors have led to efforts to identify new pharmacological agents to be used in the molecular dissection of auxin transport processes (27). Gravacin (3-(5-[3,4-dichlorophenyl]-2-furyl)acrylic acid) was originally identified in a screen for compounds that inhibit seedling gravitropism (28) and was later shown to inhibit the ABCB19 auxin transporter (20). However, gravacin has considerable nonspecific activity, as it also inhibits the trafficking of the vacuolar marker δ TIP (28). A more promising inhibitor of ABCB auxin transporters is 2-[4-(diethylamino)-2-hydroxybenzoyl]benzoic acid, although some of the phenotypes of 2-[4-(diethylamino)-2-hydroxybenzoyl]benzoic acid-treated plants resemble those observed in the pin1 mutant, suggesting that this inhibitor may have indirect effects on auxin transport in planta (29). The molecular structures of IAA and NAA are recognized by both auxin signaling and transport systems. Ideal specific inhibitors of the auxin transport system would be those that are not recognized by auxin signaling mechanisms. Here we show that 5-alkoxy-IAA and 7-alkoxy-NAA auxin analogs inhibit polar auxin transport streams and auxin-dependent tropic responses in maize and Arabidopsis. However, these alkoxy-auxins have no apparent effect on auxin-responsive gene expression mediated by the SCFTIR1 pathway, indicating that they do not bind the TIR1/AFB-AuxIAA composite auxin receptors. They also had no apparent effect on the subcellular trafficking of PIN proteins. The alkoxy-auxin analogs also inhibited the auxin transport activities of PIN, ABCB, and AUX1 transporters expressed in yeast cells. The activity and structure of these auxin analogs suggest that they are specific auxin transport inhibitors that will be valuable tools for use in plant biology research.
EXPERIMENTAL PROCEDURES
Synthesis of Chemicals
5-Alkoxy-indole 3-acetic acids (1a–7a) were synthesized from 5-hydroxy-indole 3-acetic acid methyl ester by reaction with alkyl iodide or aryl bromide and successive alkaline hydrolysis of methyl ester with aqueous sodium hydroxide. 7-Alkoxy-napthalene 1-acetic acids (1b–7b) were synthesized from alkylation and hydrolysis of 7-hydroxy naphthalene 1-acetic acid ethyl ester prepared from 7-methoxy-1-tetralone. For detailed information of the synthesis of alkoxy-auxin analogs, the scheme of chemical reactions, the synthetic procedures, and the IR, NMR, and MS spectral data, see supplemental data.
Plant Growth Conditions
Arabidopsis seedlings of ecotype Columbia (Col-0) were used for all experiments unless otherwise stated. Arabidopsis aux1–7 and eir1/pin2 mutants were obtained from the Arabidopsis Biological Resource Center. Arabidopsis seedlings were grown on germination (GM) medium (30) containing 1.5% sucrose and 1.4% agar under continuous white light or as otherwise stated. The PIN1:GFP-2 (31) gene was subcloned under control of cauliflower mosaic virus 35S promoter and the NOS terminator. The 35S::PIN1-GFP vector was transformed into Arabidopsis wild-type plants by the floral dip method. Transformants were selected on GM medium containing 30 μg/ml of kanamycin. Homozygous lines were identified in the T3 generation, and the T4 homozygous line was used for analysis. Seeds of maize (Zea mays L. cv. Golden Cross Bantam 70) were germinated at 25 °C under red light for 2 days and then in darkness for 1 day as described previously (32). Coleoptile tip sections (3 mm) were used for determination of polar auxin transport assay. Coleoptile segments 20 mm in length were used for gravitropic assay (33).
Gravitropic and Phototropic Assays
For Arabidopsis gravitropism and phototoropism assays, seeds were surface-sterilized and sown in plastic culture dishes containing half-strength Okada and Shimura (OS) medium (34) containing 1.5% agar or GM medium containing 1.5% sucrose and 1.4% agar. The medium used for assays and the concentration of chemicals are indicated in figure legends. The seeds were kept in a cold room at 4 °C for 3 or 4 days followed by exposure to red light for 4 h to induce uniform germination. After germination had been induced, the dishes were incubated vertically at 22 °C. White light was provided by a white fluorescent lamp (model FL20SSW/18; National, Tokyo, Japan). Blue and red light were provided by light-emitting diodes (470 ± 30 and 660 ± 30 nm, respectively), as described previously (35). Seedling images were generated using an ES-8500 image scanner (SEIKO-EPSON, Suwa, Japan) and stereomicroscope (MZ-16F: Leica, Wetzlar, Germany and SZX9: Olympus, Tokyo, Japan), from which hypocotyl and root curvatures were then measured. Maize coleoptile gravitropic responses were conducted according to the methods described in Ref. 33. Briefly, inhibitors were dissolved in 10 mm potassium phosphate buffer (pH 6.7) and then injected into the inner surface of the coleoptile segments (20 mm long excised from the seedling). The base of each coleoptile was clamped in 1% agar and tilted horizontally. The photos of coleoptiles were taken after 120 min.
GUS Reporter Assays
For quantitative measurement of GUS enzyme activity, seedlings (n = 15–20) were homogenized in an extraction buffer as previously described (36). After centrifugation to remove cell debris, GUS activity was measured with 1 mm 4-methyl umbelliferyl β-d-glucuronide as a fluorogenic substrate at 37 °C. Protein concentration was determined by Bradford protein assay (Bio-Rad). The experiments were repeated at least three times with three replications. For histochemical staining, the seedlings were washed with GUS staining buffer (36) and transferred to the GUS staining buffer containing 1 mm 5-bromo-4-chloro-3-indolyl β-d-glucuronide and incubated at 37 °C until sufficient staining was observed. For comparative assays, the same development time was used for all samples.
Aux/IAA Fusion Protein Degradation Assays
The 7-day-old HS::AXR3NT-GUS and HS::axr3–1NT-GUS transgenic seedlings (37) were incubated in liquid GM medium for 2 h at 37 °C and then transferred into liquid GM medium at 24 °C. After 20 min at 24 °C, inhibitors and 2 μm NAA were added into the medium at the indicated concentrations. The seedlings were incubated at 24 °C for another 30 min, then immersed with 70% cold acetone, and washed with water. GUS activity was histochemically stained until sufficient staining was developed.
Auxin Transport Activity of PIN, ABCB, and AUX1 Heterologously Expressed in Yeast
Auxin transport assays of AUX1, PIN, and ABCB transporters were conducted in Schizosaccharomyces pombe as previously described (14, 38). All chemicals were purchased from Sigma unless otherwise specified. Briefly, S. pombe cells were grown to A600 ∼2.0 in Edinburgh minimal medium containing 15 μm thiamine. The thiamine was removed by washing twice with Edinburgh minimal medium, and cells were transferred to fresh Edinburgh minimal medium and incubated for 19 h (final A600 ∼ 2) to induce the expression of proteins. [3H]IAA transport assays were performed as described previously (14, 38). Inhibitors were prepared as ethanol solutions and added together with [3H]IAA.
Plant Polar Auxin Transport Assays
The Arabidopsis polar auxin transport assay were performed as described previously (16, 19, 39–41). Briefly, 0.01 pmol of [3H]IAA was applied to shoot apical tissues in a 10-nl micro-droplet of 1 μm [3H]IAA with seedlings placed on a discontinuous filter paper system. Transport of radiolabeled auxin to the root shoot transition zone (2-mm segments of 10 seedlings) was measured by scintillation counting. Inhibitors were added together with [3H]IAA. For the maize coleoptile polar auxin transport assays, maize coleoptile tips (3 mm) that were excised from the seedling and inhibitors in potassium phosphate buffer solution were injected into the inner surface of the coleoptile tip (shown in Fig. 4A). The coleoptile tip was then placed on agar block. The block was replaced after every 30-min intervals. The IAA content transported from the coleoptile tip to agar block was measured by gas chromatography-mass spectrometry (33, 42).
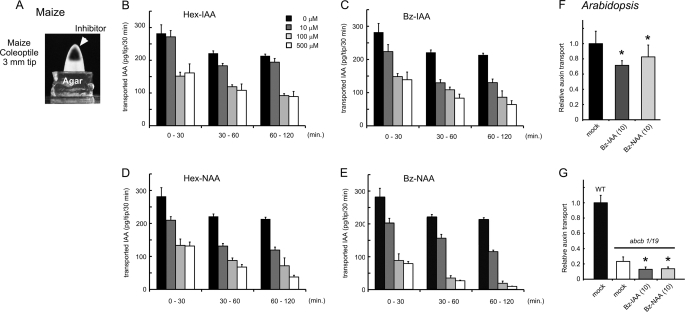
FIGURE 4.
Effects of 5-alkoxy-IAA and 7-alkoxy-NAA on polar auxin transport in Arabidopsis seedling and maize coleoptile. A–E, inhibitory effects of alkoxy-auxin on polar auxin transport in maize coleoptile. A, maize coleoptile tip (3 mm) was excised and then the inhibitor solution was loaded into the inner surface of the coleoptile. The transported IAA from the coleoptile tip was trapped in the receiver agar block. The block was replaced at 30-min intervals and the transported IAA content in the block was measured by GC-MS (33). Error bars (n = 3) indicate the mean ± S.E. F and G, inhibitory effects of alkoxy-auxin on polar auxin transport from the Arabidopsis shoot apex to root in wild-type and the abcb1/acbcb19 mutant. F, 10 μm Bz-IAA (t test p = 0.04) and Bz-NAA (p = 0.02) reduced the 3H-labeled IAA transport levels to 65 and 82% of the untreated plant, respectively. G, in the abcb1/19 mutant, the [3H]IAA movement was reduced to 23% of the level of wild-type (p < 0.001). 10 μm Bz-IAA (p = 0.08) and Bz-NAA (p = 0.09) further reduced the 55 and 58% levels of the [3H]IAA transport in the abcb1/abcb19 mutant. Error bars represent the S.D. (n = 5). Asterisks indicate p < 0.001.
Subcellular Imaging of PIN2-GFP Fusion Proteins
Inhibitors were exogenously applied by incubation of 5-day-old Arabidopsis seedlings expressing ProPIN2:PIN2-GFP (43) in GM liquid medium supplemented with TIBA (25 μm) or alkoxy-auxin analogs (10 μm). Control treatments contained an equivalent amount of solvent (dimethyl sulfoxide). Seedlings were pre-treated with each inhibitor for 30 min. Then, 50 μm brefeldin A (BFA) was added to the medium and incubated for an additional 2 h. PIN2-GFP fluorescence was imaged by confocal laser-scanning microscopy (FV1000; Olympus, Japan). Conversely, the seedling was initially treated with 50 μm BFA for 2 h. Then after washout of BFA, the seedling was incubated in GM medium containing inhibitors for an additional 2 h. The PIN2-GFP image of the root cells was obtained.
RESULTS
Selected Alkoxy-auxin Analogs Inhibit Root Gravitropism without Modulating SCFTIR1 Auxin Signaling
The effects of chemically modified forms of IAA and NAA on auxin-related growth phenotypes of Arabidopsis seedlings were examined. Inhibition of root gravitropism is a common and characteristic activity of auxin transport inhibitors. 5-Alkoxy-IAA (5a–7a) and 7-alkoxy NAA (3b–7b) derivatives inhibited of root gravitropism (Fig. 1, A and B), with a group of 7-alkoxy-NAA compounds (5b–7b) showing the greatest effect (Fig. 1B and supplemental Fig. S1).
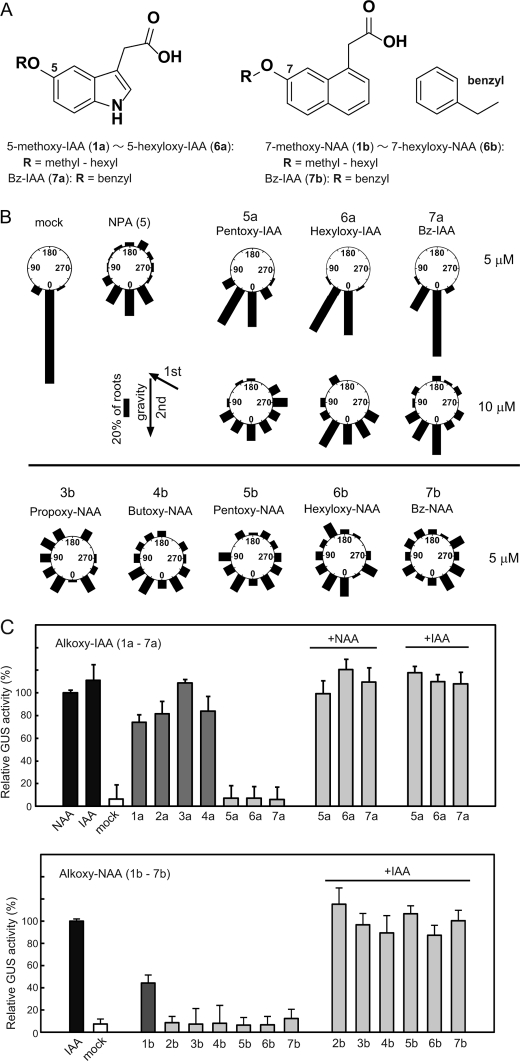
FIGURE 1.
Effects of 5-alkoxy-IAA and 7-alkoxy-NAA on gravitropic response and auxin-responsive DR5::GUS reporter gene expression. A, chemical structures of 5-alkoxy-IAA, 1a-7a; and 7-alkoxy-NAA, 1b-7b. B, inhibitory activity of 5-alkoxy-IAA (5a–7a) and 7-alkoxy-NAA (3b–7b) on Arabidopsis root gravitropism. Arabidopsis seedling was cultured vertically on GM agar medium for 5 days under white light. The 5-day-old seedlings were grown in the first gravity direction (1st g) on vertical GM agar medium with chemical in the dark for 24 h and then rotated at an angle of 135° (2nd g) and cultured for an additional 24 h. The frequency (%) of root growth direction at intervals of 30° are represented by the lengths of the bars. About 50 seedlings were measured in each experiment. 5 μm NPA was used for positive control. C, auxin and antiauxin activity of alkoxy-auxins (1a–7b). The 5-day-old Arabidopsis auxin-responsive DR5::GUS reporter line was incubated in the liquid germination (GM) medium containing 20 μm alkoxy-auxins with or without 2 μm IAA or NAA for 5 h. The auxin-induced GUS reporter enzyme activity was fluorometrically determined. Relative GUS activity induced by 2 μm auxin was shown as 100% value. Error bars represent S.D. (n = 15–20).
However, antiauxins such as p-chlorophenoxyisobutylic acid, yokonolide B, terfstatin A, and α-alkyl-IAA (BH-IAA) also inhibit SCFTIR1 auxin signaling to reduce the root gravitropism (36, 44–46). The Arabidopsis TIR1/AFB F-box components of SCF E3 ubiquitin-ligase complexes function coordinately with Aux/IAA proteins as auxin receptors. Auxin functions as a molecular glue to increase interactions between TIR1/AFBs and Aux/IAAs, which repress auxin-responsive gene expression. The result of this interaction is ubiquitination and subsequent degradation of Aux/IAAs and activation of auxin responsive gene expression.
The effects of 5-alkoxy-IAA (1a–7a) and 7-alkoxy-NAA (1b–7b) on this receptor system were systematically evaluated at the molecular level using the auxin-responsive DR5::GUS reporter that is tightly regulated by the SCFTIR1 auxin signaling pathway (47). Auxin-induced GUS activity was monitored by fluorometrically quantitative measurements using a fluorogenic substrate. Like IAA and NAA, 5-methoxy-IAA (1a) to 5-butoxy-IAA (4a) induced DR5::GUS expression at 20 μm (Fig. 1C). In contrast, 5-pentoxy-IAA (5a), 5-hexyloxy-IAA (6a), and 5-benzyloxy-IAA (7a) did not, indicating that these IAA analogs do not activate the SCFTIR1 auxin signaling pathway. With the exception of 7-methoxy-NAA (1b), which activated DR5::GUS expression, 7-alkoxy-NAA analogs (2b–7b) had no effect on DR5::GUS expression. Furthermore, in the presence of auxin, 5-alkoxy-IAA (5a–7a) and 7-alkoxy-NAA (2b–7b) did not inhibit DR5::GUS expression at 20 μm (Fig. 1C), indicating that these analogs are not antiauxins. However, Arabidopsis seedlings treated with 50 μm 7-ethoxy-NAA (2b) did exhibit some alterations in auxin-related phenotypes such as primary root inhibition and root hair promotion (supplemental Fig. S1A), suggesting that this analog has weak auxin activity.
Based on these results, 5-hexyloxy-IAA (6a: Hex-IAA), 7-hexyloxy-NAA (6b: Hex-NAA), 5-benzyloxy-IAA (7a: Bz-IAA), and 7-benzyloxy NAA (7b: Bz-NAA) were selected for further detailed evaluation. Benzyloxy-IAA (7a) and benzyloxy-NAA (7b) showed no effects on the auxin-regulated promoter activity of native auxin-responsive Aux/IAA genes (IAA3, IAA7, IAA12, IAA13, IAA14, and IAA19) regulated by the SCFTIR1 pathway (supplemental Fig. S2). These results are consistent with modeling of the docking of these compounds to the TIR1-Aux/IAA crystal structure, which indicates that alkoxy-auxins with bulky alkoxy chains (7a and 7b) could not fit into the auxin binding pocket of the TIR1 auxin receptor (supplemental Fig. S2).
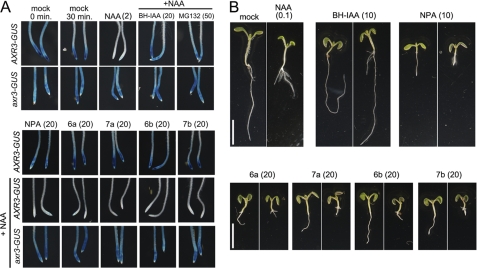
Active auxins destabilize Aux/IAA proteins, and this interaction can be detected using the HS::AXR3NT-GUS fusion of the GUS reporter to the N terminus of IAA7/AXR3 containing the interaction/stability domain II expressed under control of a heat shock promoter in Arabidopsis (37). The degradation of the AXR3-GUS fusion protein is rapidly enhanced by auxin (e.g. 2 μm NAA) via activation of the SCFTIR1 pathway. As a control, HS::axr3–1NT-GUS, in which a mutation in domain II confers resistance to auxin-induced degradation of the fusion protein, was used. After heat induction, the seedlings were treated with alkoxy-auxin derivatives in the presence of 2 μm NAA. After a 30-min incubation without NAA, activity of the AXR3NT-GUS fusion protein was observed and NAA accelerated the degradation of fusion protein as previously described (Fig. 2A) (37). The TIR1-specific auxin antagonist α-tert-butoxycarbonyl aminohexyl-IAA (BH-IAA) (46) and the proteasome inhibitor MG132 blocked the NAA-enhanced degradation of the fusion protein. Alkoxy-auxins (6a, 7a, 6b, and 7b) and NPA had no inhibitory effects on the auxin-regulated degradation of AXR3 fusion proteins. Additionally, alkoxy-auxins did not affect the stability of the axr3–1NT-GUS fusion protein, suggesting that the observed effects were on Aux/IAA protein degradation, not expression or translation of the proteins.
FIGURE 2.
Effects of 5-alkoxy-IAA and 7-alkoxy-NAA on auxin-dependent degradation of Aux/IAA protein and auxin-inhibited root growth of Arabidopsis seedling. A, effects of 5-hexyloxy-IAA (6a: Hex-IAA), 7-hexyloxy-NAA (6b: Hex-NAA), 5-benzyloxy-IAA (7a: Bz-IAA), 7-benzyloxy NAA (7b: Bz-NAA), BH-IAA (TIR1 auxin antagonist), and MG132 (proteasome inhibitor) on IAA17/AXR3-GUS fusion protein degradation. After heat shock of the 7-day-old HS::AXR3NT-GUS and HS::axr3NT-GUS transgenic seedlings for 2 h at 37 °C, the seedlings were incubated in 20 μm BH-IAA, 20 μm alkoxyl-auxins, or 50 μm MG132 with/without 2 μm NAA for 30 min at 24 °C. The residual GUS fusion protein was developed with histochemical staining. B, Arabidopsis seedlings were grown with the inhibitors in the absence (left panel) or presence of 0.1 μm NAA (right panel) on the GM plate solidified with 0.1% gellan gum for 6 days under continuous light. The values in parentheses present the concentration of chemicals at micromolar. Scale bar represents 5 mm.
To further analyze the antiauxinic activity of alkoxy-auxin analogs, the effects of the selected alkoxy auxins (6a, 7a, 6b, and 7b) on auxin-induced root phenotypes was investigated (Fig. 2B). As expected, NAA inhibited Arabidopsis primary root growth, but promoted lateral root formation. The alkoxy-auxins (6a–7b) and NPA inhibited primary root growth and lateral root formation. The auxin signaling antagonist BH-IAA counteracted the effects of exogenous NAA to restore normal root growth. On the contrary, alkoxy-auxins (6a–7b) and NPA enhanced the inhibitory effects of NAA on root growth, suggesting these auxin analogs increased intercellular NAA accumulations by inhibiting the NAA export. Further addition of exogenous auxin did not reduce the inhibition of root growth and gravitropsim by alkoxy-auxins (6a–7b), suggesting that these auxin analogs are not auxin biosynthesis inhibitors (48). Additionally, these results indicate that alkoxy-auxins act on PIN and ABCB auxin exporters to accumulate intercellular NAA, as artificial auxin NAA can be a substrate for PIN and ABCB exporters, but not for the AUX1 importer. In summary, these results suggest that alkoxy-auxins (6a–7b) are inactive auxin analogs for SCFTIR1 auxin signaling, because the molecule size of the analogs (6a–7b) would be so large that they could not bind to the TIR1 auxin binding site. On the other hand, the analogs (6a–7b) were active to the polar auxin transport system regulated by PIN, ABCB, and AUX1 auxin transport proteins.
Alkoxy-auxin Analogs Block the Auxin Transport Activity of PIN, ABCB, and AUX1 Expressed in S. pombe
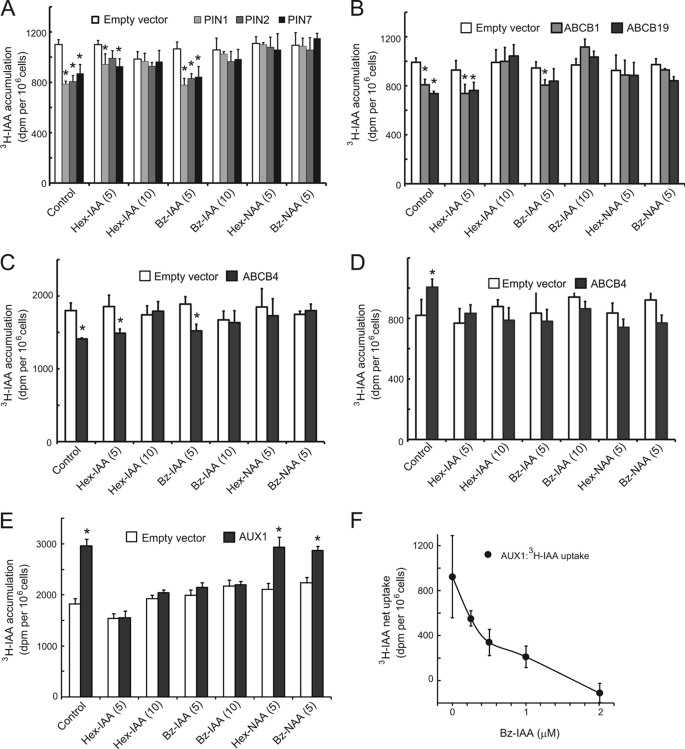
Screens of the inhibitory effects of alkoxy-auxin analogs on auxin transport mediated by recombinant PIN, ABCB, and AUX1 expressed in S. pombe indicated that the analogs could inhibit this process. Hex-IAA and Bz-IAA (6a and 7a) at 10 μm and Hex-NAA and Bz-NAA (6b and 7b) at 5 μm blocked auxin export by PIN1, -2, and -7 (Fig. 3A). Two alkoxy-NAA analogs (6b and 7b) showed greater inhibition of ABCB1 and ABCB19 export activity than alkoxy-IAA analogs (Fig. 3B). Bz-NAA (7b) at 5 μm displayed more selective inhibition of PIN proteins than ABCB proteins, with greatly decreased activity against ABCB19. In contrast, 5 μm Bz-IAA had a greater effect on ABCB proteins compared with PIN proteins. Assays of 5-pentoxy-IAA (5a) and 7-propoxy-NAA (3b) indicated that these compounds are weak auxin transport inhibitors.
FIGURE 3.
Effects of 5-alkoxy-IAA and 7-alkoxy-NAA on auxin transport activity of PIN, ABCB and AUX1 proteins expressed in yeast S. pombe. The S. pombe cells were grown for 19 h to induce the expression of transport proteins by thiamine deficiency. [3H]IAA transport assays were performed as described previously (14, 38). Alkoxy-auxin analogs were added together with [3H]IAA (50 nm). The accumulated [3H]IAA within yeast cells are measured after washing the cells. A, inhibitory effect of alkoxy-auxin, 5-hexyloxy-IAA (6a: Hex-IAA), 7-hexyloxy-NAA (6b: Hex-NAA), 5-benzyloxy-IAA (7a: Bz-IAA), and 7-benzyloxy NAA (7b: Bz-NAA) on IAA efflux transport of PIN1, PIN2, and PIN7. B, inhibitory effect of alkoxy-auxin on IAA efflux transport of ABCB1 and ABCB19. C, inhibitory effect of alkoxy-auxin on IAA efflux transport of conditional transporter ABCB4. D, inhibitory effect of alkoxy-auxin on IAA influx transport of conditional transporter ABCB4. E, inhibitory effect of alkoxy-auxin on IAA influx transport of AUX1. Error bars represent S.D. (n = 6). F, dose-dependent inhibition of AUX1 by Bz-IAA (7a). Auxin accumulation was measured at 50 nm [3H]IAA with varying concentrations of Bz-IAA (7a). Error bars represent S.D. (n = 6). Asterisks indicate significant difference (p < 0.01, Student's t test). The values in parentheses present the concentration of chemicals at micromolar.
When expressed in yeast, ABCB4 functions as a conditional auxin importer mediating uptake at short-term low concentrations and efflux at higher concentrations (14). Alkoxy-auxins (6a-7b) inhibited both IAA import and export activity of ABCB4 (Fig. 3, C and D). The IAA analogs Hex-IAA (6a) and Bz-IAA (7a) effectively blocked IAA import by the AUX1 influx transporter, but Hex-NAA (6b) and Bz-NAA (7b) showed significantly less effect (Fig. 3, E and F). Bz-IAA (7a) showed a linear dose-dependent inhibition of AUX1 transport activity and complete inhibition at 2 μm (Fig. 3F). AUX1 importer recognizes the synthetic auxin 2,4-D and natural auxin IAA, but not NAA as a substrate (13, 14). This substrate profile of AUX1 is consistent with the inhibitory profile of AUX1 by alkoxy-IAA and -NAA (6a–7b). The artificial auxin 2,4-D is incorporated into cells by the AUX1 importer and inhibits primary root growth. The auxin influx inhibitor 1-napthyloxy acetic acid restores 2,4-D-inhibited primary root growth by reducing the uptake of 2,4-D by the AUX1 importer (13, 23). In an analogous experiment, Bz-IAA, but not Bz-NAA rescued 2,4-D inhibited root growth (supplemental Fig. S3), suggesting that the alkoxy-IAA analog could reduce auxin influx transport by AUX1, but alkoxy-NAA analog could not.
Alkoxy-auxin Analogs Inhibit Auxin Transport in Maize and Arabidopsis
Alkoxy-auxin analogs inhibited polar auxin transport in two standard model systems, maize coleoptiles and Arabidopsis seedlings. Inhibitor solutions or solvent were injected into the tips of maize coleoptile segments (apical 3 mm). IAA synthesized at the apex and transported through the coleoptile segment was collected in a receiver agar block, extracted, and assayed by gas chromatography-mass spectrometry (Fig. 4A). In this assay system, NPA and BFA, a fungal inhibitor of membrane trafficking that causes internalization of PIN and other membrane proteins, are reported to have IC50 values of ∼50 and 300 μm, respectively (33). Hex- and Bz-auxin analogs at 100 μm (6a, 6b, 7a, and 7b) blocked over 50% of the IAA transport in this assay (Fig. 4, B–E). Among these analogs, Bz-NAA (7b) was the most potent inhibitor and, as expected, alkoxy-NAA analogs exhibited greater inhibition than corresponding alkoxy-IAA analogs.
In nanoscale [3H]IAA radiotracer transport assays in Arabidopsis seedlings, 3H-labeled IAA was applied at the shoot apical meristem and transported [3H]IAA was measured by excision of segments at the root shoot transition zone. Alkoxy-auxin analogs were co-applied with [3H]IAA as indicated. In wild-type, IAA transport was reduced to 65 and 82% levels by treatment of seedlings with 10 μm Bz-IAA and Bz-NAA, respectively (Fig. 4F, p < 0.001). In the abcb1 abcb19 double mutant, [3H]IAA transport was reduced to 23% of wild-type, and 10 μm Bz-IAA and Bz-NAA treatment decreased residual [3H]IAA transport by a further ∼50% (Fig. 4G, p < 0.001). Addition of a permeabilization agent (Silwet-77) to the alkoxy-auxin solutions variably increased the extent of inhibition in all cases by 20–40%. Global treatment of wild-type seedlings with 10 μm Bz-NAA or Bz-IAA during the course of transport experiments (4 h) resulted in greater reductions (to 28 and 14%, respectively). Overall, these results suggest that alkoxy-auxin derivatives exhibit similar inhibitory selectivity to that observed in yeast transport assays.
More Detailed Analysis of Bz-NAA Inhibition of Auxin Transport
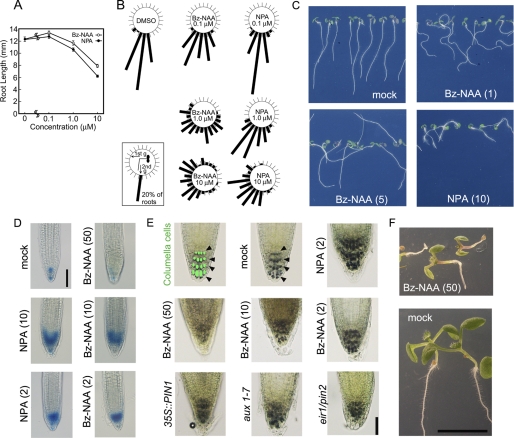
From the experiments described above, Bz-NAA emerged as the most attractive new selective auxin transport inhibitor due to its potent inhibitory activity and likely resistance to metabolism associated with IAA in planta. LC-MS analysis of methanol extracts of Arabidopsis seedlings treated for 2 h with 2 μm Bz-NAA and then transferred to control medium for 24 h readily detected residual Bz-NAA. Bz-NAA inhibited Arabidopsis root growth (Fig. 5A), gravitropism (Fig. 5, B and C), and phototropism (supplemental Fig. S4A). Bz-NAA inhibited root tropic growth responses at concentrations 10 times lower than NPA (Fig. 5B), but inhibited primary root growth to a lesser extent than NPA (Fig. 5A), presumably as a result of NPA interactions with other proteins that regulate primary root growth (21).
FIGURE 5.
Effects of Bz-NAA on root growth in Arabidopsis seedlings. A, root lengths. Seedlings were grown vertically on half-strength Okada and Shimura (OS) agar medium (34) with Bz-NAA (7b) or NPA for 5 days in white light at 100 μmol m−2 s−1. Data and error bars represent the mean ± S.E. (n = 95–100). B, root gravitropism. Before incubation, the seed's orientations were adjusted upwards as described previously (52). Seedlings were grown in the first gravity direction (1st g) on vertical OS agar medium with Bz-NAA or NPA under white light at a confluence rate of 50 μmol m−2 s−1. The 4-day-old seedlings were rotated at an angle of 90° (2nd g) and kept in the same white light condition for a further 24 h. The frequency (%) of root growth direction at intervals of 15° are represented by the lengths of the bars. About 90 seedlings were measured in each experiment. C, photograph of 5-day-old Arabidopsis wild-type seedlings (Col-0) grown vertically on OS agar medium containing Bz-NAA or NPA. D, histochemical staining of root tips in the Arabidopsis DR5::GUS line grown with Bz-NAA or NPA for 4 days. Scale bar represents 100 μm. E, effects of Bz-NAA and NPA on the organization of columella cells. Arabidopsis aux1–7, eir1/pin2, and PIN1-overexpressed mutants were cultured for 5 days on GM agar medium. Wild-type (Col) seedlings were grown with chemicals at the indicated concentrations for 5 days. Arrows indicate the columella cells visualized by lugol staining. Scale bar represents 50 μm. F, Arabidopsis wild-type seedling (Col) grown vertically for 6 days on GM agar medium containing Bz-NAA. Scale bar represents 5 mm. C–F, the values in parentheses present the concentration of chemicals at micromolar concentrations.
Under the growth conditions used in these studies, local auxin maxima reported by DR5:GUS expression are observed only in columella and meristem cells of Arabidopsis root tips (Fig. 5D) (49). In short-term treatments with Bz-NAA or NPA, both enhanced DR5::GUS expression at the root apex, suggesting that both inhibitors enhance the accumulation of endogenous IAA in the root apex by reducing auxin efflux. However, longer term or higher concentration treatments with Bz-NAA diminished the auxin-dependent organization of columella cells similar to what is seen in the eir1/pin2, aux1–7, and constitutively active 35S::PIN1 mutants (Fig. 5E) or after extended treatment with NPA ≥ 2 μm. Under these conditions, DR5::GUS expression was eliminated, the root apex was severely disrupted, and other severe defects of root development were evident (Fig. 5, E and F). These results suggest that high concentrations of Bz-NAA outcompete endogenous IAA and can be as effective as NPA treatment without the additive effects of NPA interactions with other targets.
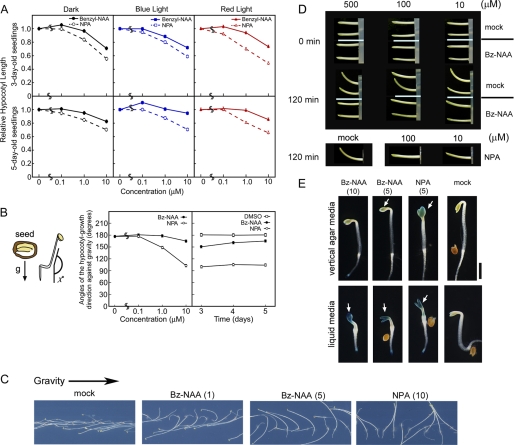
The effects of Bz-NAA on Arabidopsis hypocotyl elongation and tropic responses were assessed in 3- and 5-day-old Arabidopsis seedlings under dark, blue, and red lights. Bz-NAA inhibited hypocotyl elongation in both light and dark as well as gravitropic growth, but to a lesser extent than NPA (Fig. 6, A–C, and supplemental Figs. S5 and S6), presumably due to lower permeation into hypocotyl tissues. The inhibitory effect of Bz-NAA on gravitropism was also observed in maize coleoptiles (Fig. 6D). Other alkoxy-auxins, Hex-IAA (6a), Bz-IAA (7a), and Hex-NAA (6b) showed similar inhibitory effects on hypocotyl tropism. (supplemental Fig. S6, A and B). Three auxin transport mutants, aux1, pin3, and abcb19, have been shown to exhibit altered rates of gravitropic bending (12, 50, 51). As would be expected from a spectrum of auxin transport inhibition, Bz-NAA inhibited gravitropic hypocotyl bending in all of these mutants (supplemental Fig. S5).
FIGURE 6.
Effects of Bz-NAA on hypocotyl growth in Arabidopsis seedlings and maize coleoptile. Seed orientations were adjusted upward in analyses of A and horizontal in B and C as described previously (50). All data and error bars represent the mean ± S.E. (n = 84–102). A, relative hypocotyl lengths. Seedlings were grown on vertical OS agar medium with Bz-NAA or NPA for 3 (upper panels) or 5 days (bottom panels). Seedlings were grown in dark, continuous red light, or blue light conditions at 1.0 μmol m−2 s−1. B, angles (χ°) of the hypocotyl-growth direction against gravity (g) of 5-day-old etiolated seedlings. The seedlings were grown in the dark on vertical agar medium with Bz-NAA or NPA. When the orientation of the seeds in vertical agar medium was such that the hook of the seed embryo was located horizontally, the NPA-treated seedlings show horizontal growth of their hypocotyls according to the seed orientation, and the mock-treated seedlings show the upward growth of their hypocotyls according to negative gravitropism, as described previously (50). C, gravitropic response of 5-day-old Arabidopsis wild-type seedling (Col) vertically grown on OS agar medium with Bz-NAA or NPA. The values in parentheses present the chemicals at micromolar concentrations. D, effects of Bz-NAA and NPA on gravitropic curvature of the maize coleoptile segment (20 mm). The inhibitor solution at the indicated concentration was loaded into the inside of a 2-mm tip of coleoptile and then the coleoptile anchored on agar was cultured vertically for 30 min. The coleoptiles are tilted horizontally to observe the gravitropic response. E, histochemical staining of Arabidopsis DR5::GUS etiolated seedlings grown on vertical GM agar medium or GM liquid medium supplemented with Bz-NAA or NPA for 3 days. C–E, the values in parentheses present the concentration of chemicals at micromolar.
However, Bz-NAA (10 μm) did not affect Arabidopsis hypocotyl phototropism (supplemental Fig. S4, B and C), although it induced apical hook opening in etiolated seedlings as is seen with 1 μm NPA (supplemental Fig. S7). Analysis of DR5::GUS expression in seedlings grown on vertical agar indicated that NPA induces a greater auxin accumulation in the shoot apex and cotyledons than Bz-NAA treatment (Fig. 6E, upper panels) (52). However, in Arabidopsis seedlings grown in liquid culture where incorporation efficiency of chemicals is greatly enhanced, Bz-NAA induced auxin accumulation in the shoot apex and cotyledons to the same extent as NPA (Fig. 6E, lower panels). Gravitropic bending assays of maize coleoptiles with inhibitors injected into the apical subdermal layers showed similar gravitropic assay responses to both Bz-NAA and NPA (Fig. 6D) consistent with decreased permeation and/or incorporation of the alkoxy-auxin inhibitors into target tissues.
Alkoxy-auxin Analogs, Bz-IAA and Bz-NAA did Not Affect Subcellular Trafficking of PIN Proteins
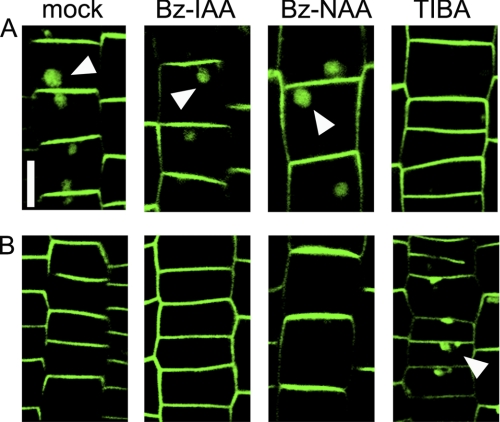
High concentrations of the classical auxin transport inhibitors TIBA and PBA alter subcellular trafficking and plasma membrane localization of PIN proteins. This effect can be visualized by inclusion of these inhibitors in washout buffers after BFA-induced internalization of PIN1 or PIN2 (25). In the presence of the inhibitors, PIN proteins are not restored to the plasma membrane and are retained in internal compartments. Similarly, co-administration of TIBA and BFA blocks internalization of the PIN2-GFP signal (43). PIN2-GFP internalization was not blocked by co-administration of alkoxy-auxins with BFA, but was blocked by TIBA (Fig. 7A). Similarly, 2 h treatment with BFA resulted in PIN2-GFP internalization, and after the washout of BFA, this PIN2-GFP protein in BFA bodies was relocalized to the plasma membrane (Fig. 7B). Bz-IAA and Bz-NAA did not alter this PIN relocalization from BFA bodies to the plasma membrane, whereas TIBA did inhibit relocalization (Fig. 7B). These results suggest that Bz-IAA and Bz-NAA do not alter cellular trafficking mechanisms and that these inhibitors can be used when effects on trafficking must be avoided.
FIGURE 7.
Bz-IAA and Bz-NAA did not affect subcellular trafficking of PIN proteins. A, Arabidopsis root expressing PIN2-GFP fusion protein was incubated in medium containing 10 μm alkoxy-auxin or 25 μm TIBA for 30 min and then 50 μm BFA was added to the same medium to relocate a PIN2-GFP protein into the BFA bodies. B, the Arabidopsis PIN2-GFP line was incubated for 2 h in medium containing 50 μm BFA to induce BFA bodies, and the root was then treated with 10 μm alkoxy-auxin or 25 μm TIBA for 2 h in the medium. GFP fluorescent was imaged with confocal microscope. Arrows represent the BFA bodies and the bar indicates 10 μm.
DISCUSSION
The results presented here indicate that alkoxy-auxin analogs are selective auxin transport inhibitors that can be used to overcome some of the nonspecific effects of inhibitors currently in use. Alkoxy-auxin transport inhibitors can be particularly useful as they do not appear to directly affect SCFTIR1 auxin signaling. The relative activities of inhibitors in this class are also consistent with observed specificities of the AUX1, PIN, and ABCB auxin transporters. For instance, AUX1 exhibits a preference for IAA and 2,4-D over NAA (13, 23), and Bz-IAA (7a) inhibits AUX1 transport, but Bz-NAA (7b) does not. Considering the structural analogy to auxin, alkoxy-auxins would be recognized at the transport site of auxin transporters and therefore, the selective inhibition of alkoxy-NAA was observed on PIN and ABCB rather than AUX1.
As is the case with all pharmacological inhibitors, it is likely that nonspecific and low-affinity targets will be discovered for alkoxy-auxin transport inhibitors in plants. NPA, 1-(2′-carboxyphenyl)-3-phenylpropane-1,3-dione, and 2-[4-(diethylamino)-2-hydroxybenzoyl]benzoic acid target the highly conserved second nucleotide binding domain of ABCB transporters, suggesting that some degree of nonspecific interaction with other ABC transporters is possible (29). Gravacin, TIBA, and PBA have more general effects on membrane protein trafficking (28, 53). As is illustrated by NPA inhibition of the metalloprotease APM1 (21), interactions with nonspecific targets tend to increase with concentration.
However, of the classical (NPA, TIBA, 1-(2′-carboxyphenyl)-3-phenylpropane-1,3-dione, and PBA) and recently characterized (2-[4-(diethylamino)-2-hydroxybenzoyl]benzoic acid and gravacin) auxin efflux inhibitors (24, 54), only TIBA is a competitive inhibitor but TIBA has weak auxin activity. As such, alkoxy-auxins provide a promising new class of selective auxin transport inhibitors with advantages of the relative substrate specificity even with increased concentration. As alkoxy-auxins also do not interfere with PIN protein trafficking and SCFTIR1 auxin signaling, their activity in planta can be presumed to be independent of impacts on these mechanisms. The alkoxy-NAA analogs also appear to be relatively resistant to degradative mechanisms during the course of standard growth experiments. These auxin transport inhibitors are promising tools for plant biology and could also lead to development of agrochemicals for crop improvement.
Supplementary Material
The work was supported by a grant from Japan Ministry of Education, Culture, Sports, Science and Technology (to K. H.) and United States Department of Energy Grant DE-FG02-06ER15804 (to A. S. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- NAA
- naphthalene 1-acetic acid
- IAA
- indole 3-acetic acid
- 2,4-D
- 2,4-dichlorophenoxy acetic acid
- Bz
- benzyloxy
- Hex
- hexyloxy
- NPA
- 1-naphthylphthalamic acid
- PBA
- pyrenoyl benzoic acid
- TIBA
- 2,3,5-triiodobenzoic acid
- BFA
- brefeldin A
- OS
- Okada and Shimura medium.
REFERENCES
- 1. Woodward A. W., Bartel B. (2005) Ann. Bot. 95, 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reinhardt D., Mandel T., Kuhlemeier C. (2000) Plant Cell 12, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reinhardt D., Pesce E. R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. (2003) Nature 426, 255–260 [DOI] [PubMed] [Google Scholar]
- 4. Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003) Cell 115, 591–602 [DOI] [PubMed] [Google Scholar]
- 5. Heisler M. G., Ohno C., Das P., Sieber P., Reddy G. V., Long J. A., Meyerowitz E. M. (2005) Curr. Biol. 15, 1899–1911 [DOI] [PubMed] [Google Scholar]
- 6. Scarpella E., Marcos D., Friml J., Berleth T. (2006) Genes Dev. 20, 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friml J. (2003) Curr. Opin. Plant Biol. 6, 7–12 [DOI] [PubMed] [Google Scholar]
- 8. Massa G. D., Gilroy S. (2003) Plant J. 33, 435–445 [DOI] [PubMed] [Google Scholar]
- 9. Zazimalova E., Murphy A. S., Yang H., Hoyerova K., Hosek P. (2010) Cold Spring Harbor Perspect. Biol. 2, a001552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrásek J., Friml J. (2009) Development 136, 2675–2688 [DOI] [PubMed] [Google Scholar]
- 11. Titapiwatanakun B., Murphy A. S. (2009) J. Exp. Bot. 60, 1093–1107 [DOI] [PubMed] [Google Scholar]
- 12. Marchant A., Kargul J., May S. T., Muller P., Delbarre A., Perrot-Rechenmann C., Bennett M. J. (1999) EMBO J. 18, 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y., Hammes U. Z., Taylor C. G., Schachtman D. P., Nielsen E. (2006) Curr. Biol. 16, 1123–1127 [DOI] [PubMed] [Google Scholar]
- 14. Yang H., Murphy A. S. (2009) Plant J. 59, 179–191 [DOI] [PubMed] [Google Scholar]
- 15. Murphy A. S., Hoogner K. R., Peer W. A., Taiz L. (2002) Plant Physiol. 128, 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geisler M., Blakeslee J. J., Bouchard R., Lee O. R., Vincenzetti V., Bandyopadhyay A., Titapiwatanakun B., Peer W. A., Bailly A., Richards E. L., Ejendal K. F., Smith A. P., Baroux C., Grossniklaus U., Müller A., Hrycyna C. A., Dudler R., Murphy A. S., Martinoia E. (2005) Plant J. 44, 179–194 [DOI] [PubMed] [Google Scholar]
- 17. Bouchard R., Bailly A., Blakeslee J. J., Oehring S. C., Vincenzetti V., Lee O. R., Paponov I., Palme K., Mancuso S., Murphy A. S., Schulz B., Geisler M. (2006) J. Biol. Chem. 281, 30603–30612 [DOI] [PubMed] [Google Scholar]
- 18. Blakeslee J. J., Bandyopadhyay A., Lee O. R., Mravec J., Titapiwatanakun B., Sauer M., Makam S. N., Cheng Y., Bouchard R., Adamec J., Geisler M., Nagashima A., Sakai T., Martinoia E., Friml J., Peer W. A., Murphy A. S. (2007) Plant Cell 19, 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geisler M., Kolukisaoglu H. U., Bouchard R., Billion K., Berger J., Saal B., Frangne N., Koncz-Kalman Z., Koncz C., Dudler R., Blakeslee J. J., Murphy A. S., Martinoia E., Schulz B. (2003) Mol. Biol. Cell 14, 4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rojas-Pierce M., Titapiwatanakun B., Sohn E. J., Fang F., Larive C. K., Blakeslee J., Cheng Y., Cutler S. R., Cuttler S., Peer W. A., Murphy A. S., Raikhel N. V. (2007) Chem. Biol. 14, 1366–1376 [DOI] [PubMed] [Google Scholar]
- 21. Peer W. A., Hosein F. N., Bandyopadhyay A., Makam S. N., Otegui M. S., Lee G. J., Blakeslee J. J., Cheng Y., Titapiwatanakun B., Yakubov B., Bangari B., Murphy A. S. (2009) Plant Cell 21, 1693–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imhoff V., Muller P., Guern J., Delbarre A. (2000) Planta 210, 580–588 [DOI] [PubMed] [Google Scholar]
- 23. Parry G., Delbarre A., Marchant A., Swarup R., Napier R., Perrot-Rechenmann C., Bennett M. J. (2001) Plant J. 25, 399–406 [DOI] [PubMed] [Google Scholar]
- 24. Katekar G. F., Geissler A. E. (1980) Plant Physiol. 66, 1190–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geldner N., Friml J., Stierhof Y. D., Jürgens G., Palme K. (2001) Nature 413, 425–428 [DOI] [PubMed] [Google Scholar]
- 26. Dhonukshe P., Tanaka H., Goh T., Ebine K., Mähönen A. P., Prasad K., Blilou I., Geldner N., Xu J., Uemura T., Chory J., Ueda T., Nakano A., Scheres B., Friml J. (2008) Nature 456, 962–976 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. De Rybel B., Audenaert D., Beeckman T., Kepinski S. (2009) ACS Chem. Biol. 4, 987–998 [DOI] [PubMed] [Google Scholar]
- 28. Surpin M., Rojas-Pierce M., Carter C., Hicks G. R., Vasquez J., Raikhel N. V. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4902–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim J. Y., Henrichs S., Bailly A., Vincenzetti V., Sovero V., Mancuso S., Pollmann S., Kim D., Geisler M., Nam H. G. (2010) J. Biol. Chem. 285, 23309–23317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oono Y., Chen Q. G., Overvoorde P. J., Köhler C., Theologis A. (1998) Plant Cell 10, 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wisniewska J., Xu J., Seifertová D., Brewer P. B., Ruzicka K., Blilou I., Rouquié D., Benková E., Scheres B., Friml J. (2006) Science 312, 883–883 [DOI] [PubMed] [Google Scholar]
- 32. Mori Y., Nishimura T., Koshiba T. (2005) Plant Sci. 168, 467–473 [Google Scholar]
- 33. Nishimura T., Nakano H., Hayashi K., Niwa C., Koshiba T. (2009) Plant Cell Physiol. 50, 1874–1885 [DOI] [PubMed] [Google Scholar]
- 34. Okada K., Shimura Y. (1992) Aust. J. Plant Physiol. 19, 439–448 [Google Scholar]
- 35. Ohgishi M., Saji K., Okada K., Sakai T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2223–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamazoe A., Hayashi K., Kepinski S., Leyser O., Nozaki H. (2005) Plant Physiol. 139, 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gray W. M., Kepinski S., Rouse D., Leyser O., Estelle M. (2001) Nature 414, 271–276 [DOI] [PubMed] [Google Scholar]
- 38. Titapiwatanakun B., Blakeslee J. J., Bandyopadhyay A., Yang H., Mravec J., Sauer M., Cheng Y., Adamec J., Nagashima A., Geisler M., Sakai T., Friml J., Peer W. A., Murphy A. S. (2009) Plant J. 57, 27–44 [DOI] [PubMed] [Google Scholar]
- 39. Murphy A., Peer W. A., Taiz L. (2000) Planta 211, 315–324 [DOI] [PubMed] [Google Scholar]
- 40. Noh B., Murphy A. S., Spalding E. P. (2001) Plant Cell 13, 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Surpin M., Zheng H., Morita M. T., Saito C., Avila E., Blakeslee J. J., Bandyopadhyay A., Kovaleva V., Carter D., Murphy A., Tasaka M., Raikhel N. (2003) Plant Cell 15, 2885–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishimura T., Mori Y., Furukawa T., Kadota A., Koshiba T. (2006) Planta 224, 1427–1435 [DOI] [PubMed] [Google Scholar]
- 43. Xu J., Scheres B. (2005) Plant Cell 17, 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oono Y., Ooura C., Rahman A., Aspuria E. T., Hayashi K., Tanaka A., Uchimiya H. (2003) Plant Physiol. 133, 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hayashi K., Jones A. M., Ogino K., Yamazoe A., Oono Y., Inoguchi M., Kondo H., Nozaki H. (2003) J. Biol. Chem. 278, 23797–23806 [DOI] [PubMed] [Google Scholar]
- 46. Hayashi K., Tan X., Zheng N., Hatate T., Kimura Y., Kepinski S., Nozaki H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5632–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ulmasov T., Liu Z. B., Hagen G., Guilfoyle T. J. (1995) Plant Cell 7, 1611–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soeno K., Goda H., Ishii T., Ogura T., Tachikawa T., Sasaki E., Yoshida S., Fujioka S., Asami T., Shimada Y. (2010) Plant Cell Physiol. 51, 524–536 [DOI] [PubMed] [Google Scholar]
- 49. Casimiro I., Marchant A., Bhalerao R. P., Beeckman T., Dhooge S., Swarup R., Graham N., Inzé D., Sandberg G., Casero P. J., Bennett M. (2001) Plant Cell 13, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nagashima A., Uehara Y., Sakai T. (2008) Plant Cell Physiol. 49, 1250–1255 [DOI] [PubMed] [Google Scholar]
- 51. Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. (2002) Nature 415, 806–809 [DOI] [PubMed] [Google Scholar]
- 52. Nagashima A., Suzuki G., Uehara Y., Saji K., Furukawa T., Koshiba T., Sekimoto M., Fujioka S., Kuroha T., Kojima M., Sakakibara H., Fujisawa N., Okada K., Sakai T. (2008) Plant J. 53, 516–529 [DOI] [PubMed] [Google Scholar]
- 53. Dhonukshe P., Grigoriev I., Fischer R., Tominaga M., Robinson D. G., Hasek J., Paciorek T., Petrásek J., Seifertová D., Tejos R., Meisel L. A., Zazímalová E., Gadella T. W., Jr., Stierhof Y. D., Ueda T., Oiwa K., Akhmanova A., Brock R., Spang A., Friml J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katekar G. F., Geissler A. E. (1977) Plant Physiol. 60, 826–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.