Abstract
Diet is a major environmental factor influencing gut microbiota diversity and functionality, which might be relevant to subjects following dietary therapies. Celiac disease (CD) is an enteropathy caused by an aberrant immune response to cereal gluten proteins and the only therapy is the adherence to a gluten-free diet (GFD). In this context, a preliminary study was conducted to establish whether the GFD in itself could modify the composition and immune properties of the gut microbiota. The trial included 10 healthy subjects (30.3 years-old), which were submitted to a GFD over one month. Analysis of fecal microbiota and dietary intake indicated that numbers of healthy gut bacteria decreased, while numbers of unhealthy bacteria increased parallel to reductions in the intake of polysaccharides after following the GFD. Fecal samples of subjects under a GFD, which represent an altered microbiota, also exerted lower immune stimulatory effects on peripheral blood mononuclear cells than those of subjects on a regular gluten-containing diet. This addendum presents further discussion on the rationale behind these findings, limitations of the study and possible consequences of dietary counselling in the care process of celiac disease patients.
Key words: gut microbiota, gluten-free diet, celiac disease, immunity, probiotics, polysaccharides, prebiotics
Relationship between the Gluten-Free Diet and the Gut Microbiota
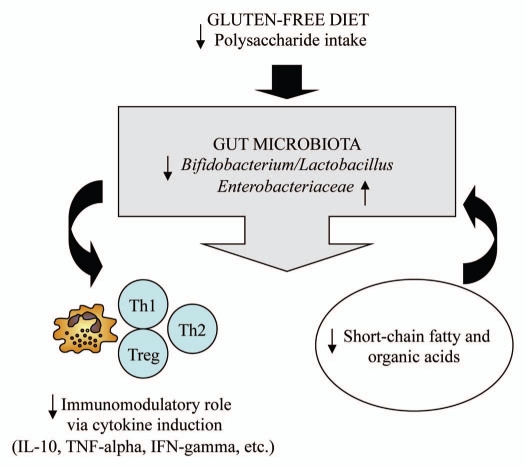
The human intestinal tract harbors a collection of beneficial bacteria (symbionts/mutualists) that perform an array of functions, including the provision of attributes not encoded in the human genome.1 This ecosystem is greatly influenced by the diet, which constitutes a major environmental factor driving bacterial diversity.2,3 Therefore, long-term dietary practices for treating food-related diseases might affect the composition of the resident microbiota and, thereby, its functional relationships with diverse host organs and tissues. In particular, intestinal bacteria constitute a constant challenge of antigens to their host that modulate mucosal immunity and the primary line of defence against antigens acquired orally. Celiac disease appeared as a result of dietary changes associated with the development of the agriculture and cereals cultivation.4 This is a chronic enteropathy caused by an aberrant immune response to cereal gluten proteins and, still the only therapy for the patients is to exclude the gluten from the diet. Although the adherence to a strict gluten-free diet (GFD) usually leads to the remission of the major clinical symptoms, nutritional deficiencies and health complications are often reported in treated patients.5–7 In addition, the microbiota of patients under a GFD is not completely restored in comparison with that of healthy subjects.8–10 In this context, we published a preliminary study to establish whether the GFD in itself could lead to modifications on the composition and immune properties of the gut microbiota.11 This study included 10 healthy subjects (30.3 years-old), who were following a GFD over one month by replacing the gluten-containing foods they usually ate with certified gluten-free foods (with no more than 20 parts per million of gluten). Analyses of fecal microbiota and dietary intakes, indicated that populations of generally regarded healthy bacteria decreased (Bifidobacterium, B. longum and Lactobacillus), while populations of potentially unhealthy bacteria increased parallel to reductions in the intake of polysaccharides (from 117 g to 63 g on average) after following the GFD. In particular, increases were detected in numbers of E. coli and total Enterobacteriaceae, which may include opportunistic pathogens.12 This evidence suggests a disruption of the delicate balance between the host and its intestinal microbiota (dysbiosis), which might favor the overgrowth of opportunistic pathogens and weaken the host defences against infection and chronic inflammation via possible alterations in mucosal immunity.13
Influence of the Gluten-Free Diet on Immune Properties of the Gut Microbiota
Cytokine production by peripheral blood mononuclear cells (PBMCs) stimulated with fecal samples of healthy individuals before and after the GFD was also evaluated to establish the possible relationships between the stimulus of the gut microbiota and the host immune function under this dietary practice.11 PBMC cultures were considered a good in vitro model for such studies since monocytes of the intestinal mucosa are known to be constantly replenished by blood monocytes.14 Immunostimulatory properties of feces, which up to 50% can be represented by bacteria, were remarkably reduced as a consequence of the GFD, inducing a significantly lower production of pro-inflammatory cytokines and chemokines (TNFα, IFNγ and IL-8) and anti-inflammatory cytokines (IL-10) in PBMCs than those collected before the GFD. It seems that GFD led to a generalized reduction of bacterial-induced cytokine production as a result of the generalized reduction of the total large intestinal bacterial load, as detected in patients under a gluten-free diet.8 The fact that the GFD led to reductions in total Bifidobacterium and B. longum numbers could also explain the reductions in the ability of fecal samples to stimulate IL-10 production, since strains of this genus and species might preferentially stimulate IL-10 secretion.15,16
Rationale Behind the Effect of GFD on the Gut Microbiota
The composition of the gut microbiota is susceptible to the influence of the diet and, especially, to the quality and quantity of ingested carbohydrates.17–19 The reductions in polysaccharide intake associated with the GFD could explain the observed changes in the microbiota, since these dietary compounds usually reach the distal part of the colon partially undigested, and constitute one of the main energy sources for commensal components of the gut microbiota.20 The genome of these bacteria encodes many enzymes specialized in the utilization of non-digestible carbohydrates, which provide these bacterial groups a competitive advantage over potentially pathogenic bacteria to colonize the intestine.2,21 Thus, the genome sequence of B. longum subsp. longum showed that more than 8% of the annotated genes were involved in carbohydrate and polysaccharide metabolism,21 which could explain the reduction of its levels after the GFD. It also seems feasible that when the growth of beneficial bacteria is not supported due to a reduced supply of their main energy sources other bacterial groups, which can be opportunistic pathogens, can overgrowth leading to intestinal dysbiosis. Within the gut ecosystem, the microbiota acts as a metabolic organ whose survival and composition is determined by a dynamic process of selection and competition. For example, survival of the commensal bacterium Bacteroides thetaiotaomicron has been demonstrated to be influenced by both the bacterial community composition and nutrient availability in a mouse model.22 In addition, products generated from polysaccharide fermentation, such as butyric acid, could play a role in this competitive process by generating a hostile environment for instance for enterobacteria. In fact, intake of complex dietary carbohydrates (e.g., dietary fiber) has been shown to influence both microbial colonization and fermentation variables in the mammalian gut. Thus, high intake of dietary fiber resulted in a greater short-chain fatty acid concentration in (e.g., acetic and butyric acids), and lower Escherichia coli counts in piglet intestine, while an opposite trend was shown with low fiber intake.23
A Possible Role of Dietary Counselling in Celiac Disease Patients
Although this preliminary study has limitations, including number of participants and the short duration of the intervention, the changes in the microbiota found in healthy subjects following a GFD were to some extent similar as those detected previously in patients after compliance with a long-term GFD. In particular, reductions in Bifidobacterium plus Lactobacillus populations relative to Gram-negative bacteria (Bacteroides and E. coli) were detected in untreated CD children and, particularly, in CD patients treated with a GFD.8 These findings indicate that this dietary therapy may contribute to reducing beneficial bacterial counts and increasing enterobacterial counts, which are microbial features associated with the disease8,10 and, therefore, it would not favor completely the normalization of the gut ecosystem in treated CD patients. On the other hand, the immune suppressive effects associated with the microbiota of subjects under a GFD may be partly beneficial for CD patients, which are prone to a Th1-biased immune response, but may also imply a defect of their defence and regulatory mechanisms against harmful bacteria and chronic inflammation. This sets up a scenario where individuals under a GFD would be more susceptible to overgrowth of harmful bacteria and infections, which might be associated with unpleasant symptoms and increased health risks. Moreover, the findings suggest that dietary counselling aimed at promoting polysaccharide and probiotic intake could be considered in the care process of treated patients in the future.
In the light of these preliminary results, further studies on the nutritional quality of the GFD and its effects on gut ecology and health in a larger population group and over longer periods are warranted. Although the evidence supports the hypothesis that dietary counselling and interventions with complex polysaccharides (prebiotics) or probiotics could benefit the health status of CD patients, this should be confirmed by specific human intervention trials in the target population group.
Figure 1.
Schematic representation of the possible interactions between the gluten-free diet and the gut microbiota.
Acknowledgements
This work was supported by grants AGL2008-01440/ALI and Consolider Fun-C-Food CSD2007-00063 from the Spanish Ministry of Science and Innovation (MICINN, Spain).
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/11868
References
- 1.Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J Biol Chem. 2009;284:24673–24677. doi: 10.1074/jbc.R109.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liszt K, Zwielehner J, Handschur M, Hippe B, Thaler R, Haslberger AG. Characterization of bacteria, clostridia and bacteroides in faeces of vegetarians using qPCR and PCR-DGGE fingerprinting. Ann Nutr Metab. 2009;54:253–257. doi: 10.1159/000229505. [DOI] [PubMed] [Google Scholar]
- 4.Meresse B, Ripoche J, Heyman M, Cerf-Bensussan N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2009;2:8–23. doi: 10.1038/mi.2008.75. [DOI] [PubMed] [Google Scholar]
- 5.Bürk K, Farecki ML, Lamprecht G, Roth G, Decker P, Weller M, et al. Neurological symptoms in patients with biopsy proven celiac disease. Mov Disord. 2009;24:2358–2362. doi: 10.1002/mds.22821. [DOI] [PubMed] [Google Scholar]
- 6.Capriles VD, Martini LA, Arêas JA. Metabolic osteopathy in celiac disease: importance of a gluten-free diet. Nutr Rev. 2009;67:599–606. doi: 10.1111/j.1753-4887.2009.00232.x. [DOI] [PubMed] [Google Scholar]
- 7.Malterre T. Digestive and nutritional considerations in celiac disease: could supplementation help? Altern Med Rev. 2009;14:247–257. [PubMed] [Google Scholar]
- 8.Nadal I, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with coeliac disease. J Med Microbiol. 2007;56:1669–1674. doi: 10.1099/jmm.0.47410-0. [DOI] [PubMed] [Google Scholar]
- 9.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008;22:232. doi: 10.1186/1471-2180-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J Clin Pathol. 2009;62:264–269. doi: 10.1136/jcp.2008.061366. [DOI] [PubMed] [Google Scholar]
- 11.De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102:1154–1160. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- 12.Hurrell E, Kucerova E, Loughlin M, Caubilla-Barron J, Hilton A, Armstrong R, et al. Neonatal enteral feeding tubes as loci for colonisation by members of the Enterobacteriaceae. BMC Infect Dis. 2009;1:146. doi: 10.1186/1471-2334-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins SM, Denou E, Verdu EF, Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig Liver Dis. 2009;27:85–89. doi: 10.1016/j.dld.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 14.De Palma G, Cinova J, Stepankova R, Tuckova L, Sanz Y. Pivotal advance: Bifidobacteria and Gram-negative bacteria differentially influence immune responses in the pro-inflammatory milieu of coeliac disease. J Leukoc Biol. 2010;87:765–778. doi: 10.1189/jlb.0709471. [DOI] [PubMed] [Google Scholar]
- 15.Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. 2007;150:531–538. doi: 10.1111/j.1365-2249.2007.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ménard O, Butel MJ, Gaboriau-Routhiau V, Waligora-Dupriet AJ. Gnotobiotic mouse immune response induced by Bifidobacterium sp. strains isolated from infants. Appl Environ Microbiol. 2008;74:660–666. doi: 10.1128/AEM.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 19.Barboza M, Sela DA, Pirim C, Locascio RG, Freeman SL, German JB, et al. Glycoprofiling bifidobacterial consumption of galacto-oligosaccharides by mass spectrometry reveals strain specific, preferential consumption of glycans. Appl Environ Microbiol. 2009;75:7319–7325. doi: 10.1128/AEM.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Graaf AA, Venema K. Gaining insight into microbial physiology in the large intestine: a special role for stable isotopes. Adv Microb Physiol. 2008;53:73–168. doi: 10.1016/S0065-2911(07)53002-X. [DOI] [PubMed] [Google Scholar]
- 21.Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc Natl Acad Sci USA. 2002;99:14422–14427. doi: 10.1073/pnas.212527599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, et al. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009;6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermes RG, Molist F, Ywazaki M, Nofrarías M, Gomez de Segura A, Gasa J, et al. Effect of dietary level of protein and fiber on the productive performance and health status of piglets. J Anim Sci. 2009;87:3569–3577. doi: 10.2527/jas.2008-1241. [DOI] [PubMed] [Google Scholar]