Abstract
A direct neural role in the regulation of immunoreactive glucagon (IRG) secretion has been investigated during stimulation of mixed autonomic nerves to the pancreas in anesthetized dogs. The responses were evaluated by measurement of blood flow and hormone concentration in the venous effluent from the stimulated region of pancreas.
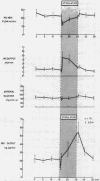
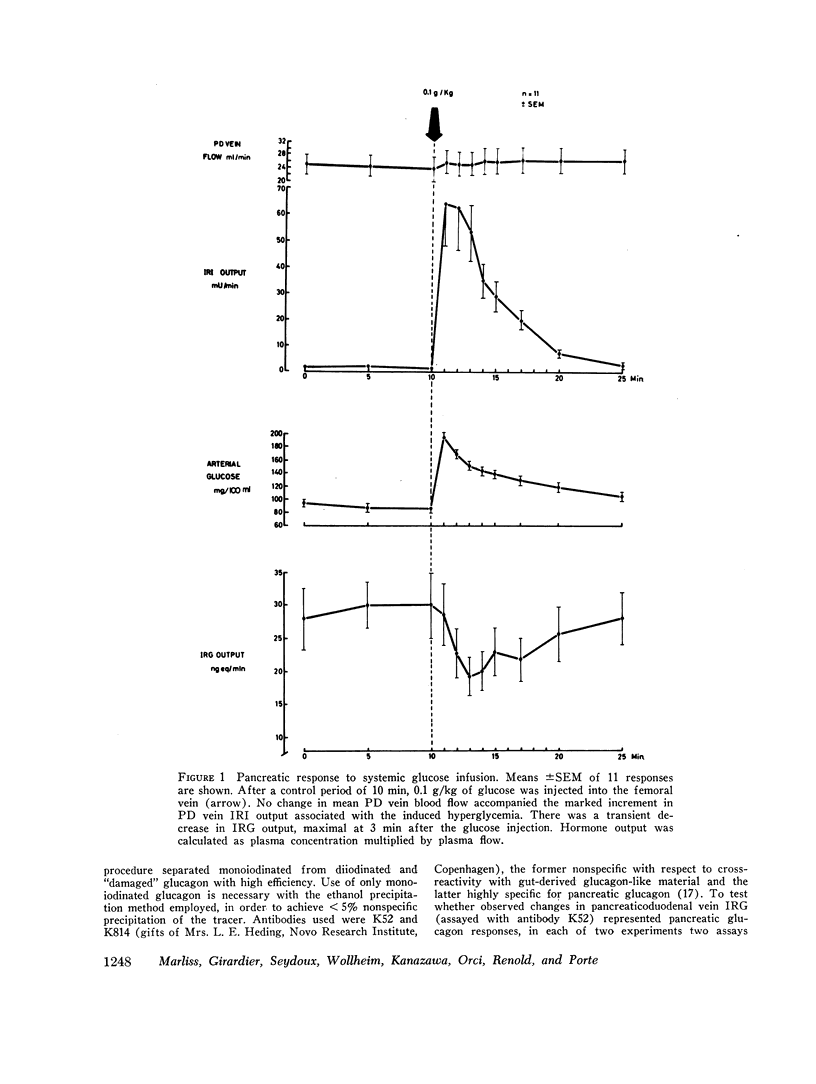
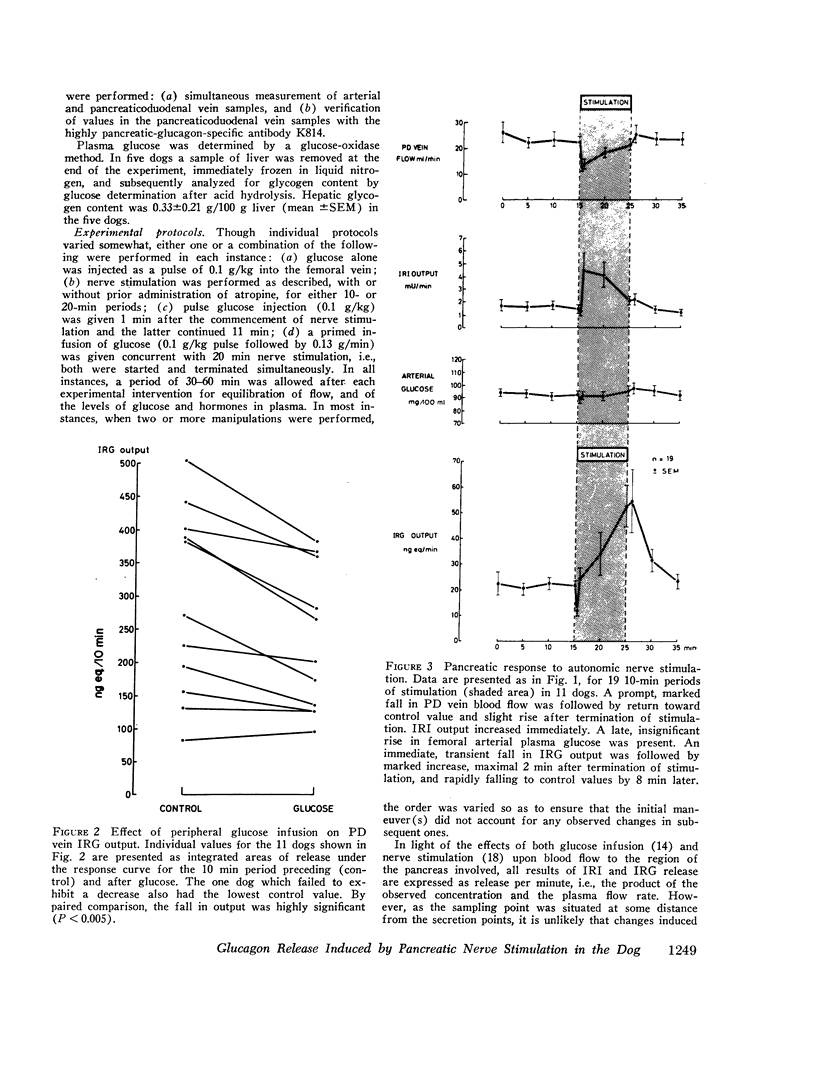
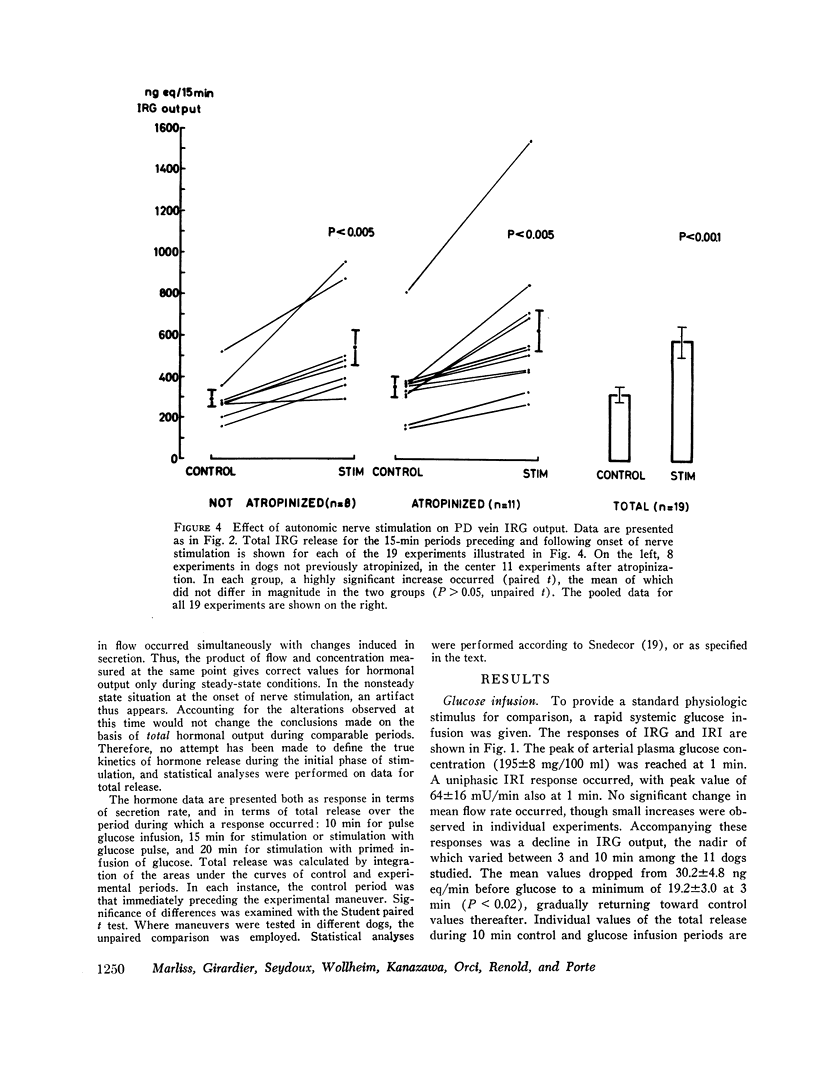
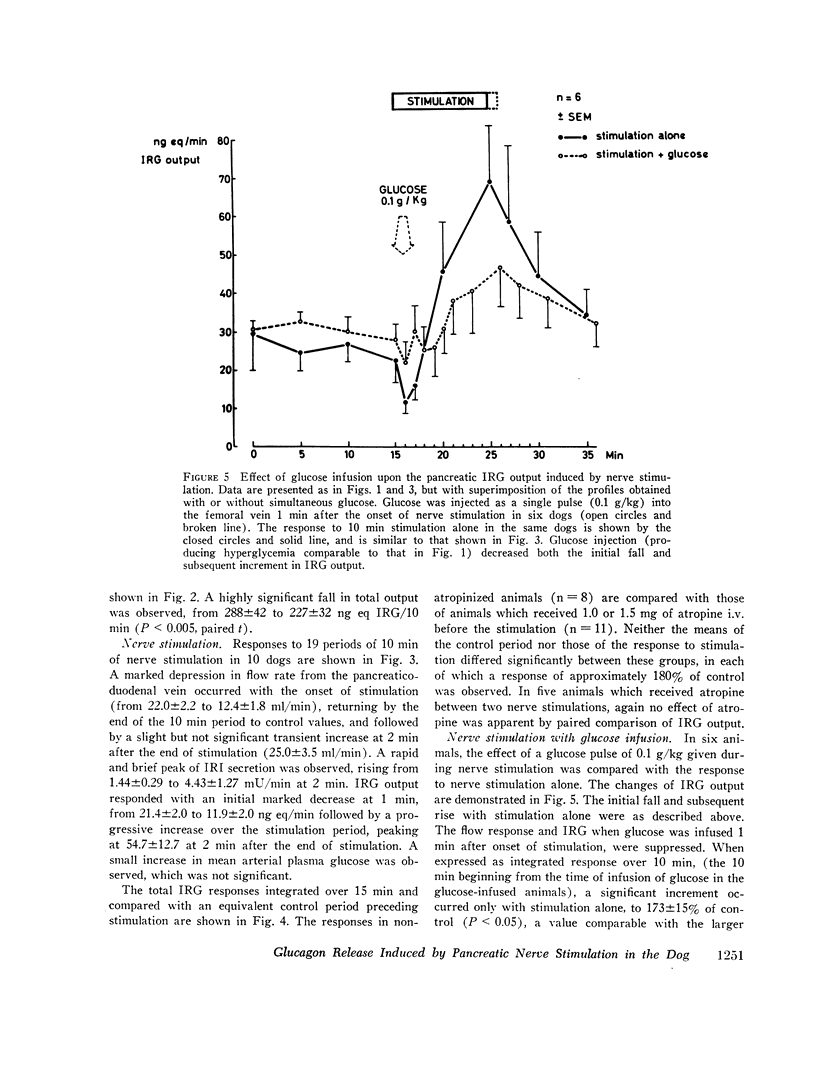
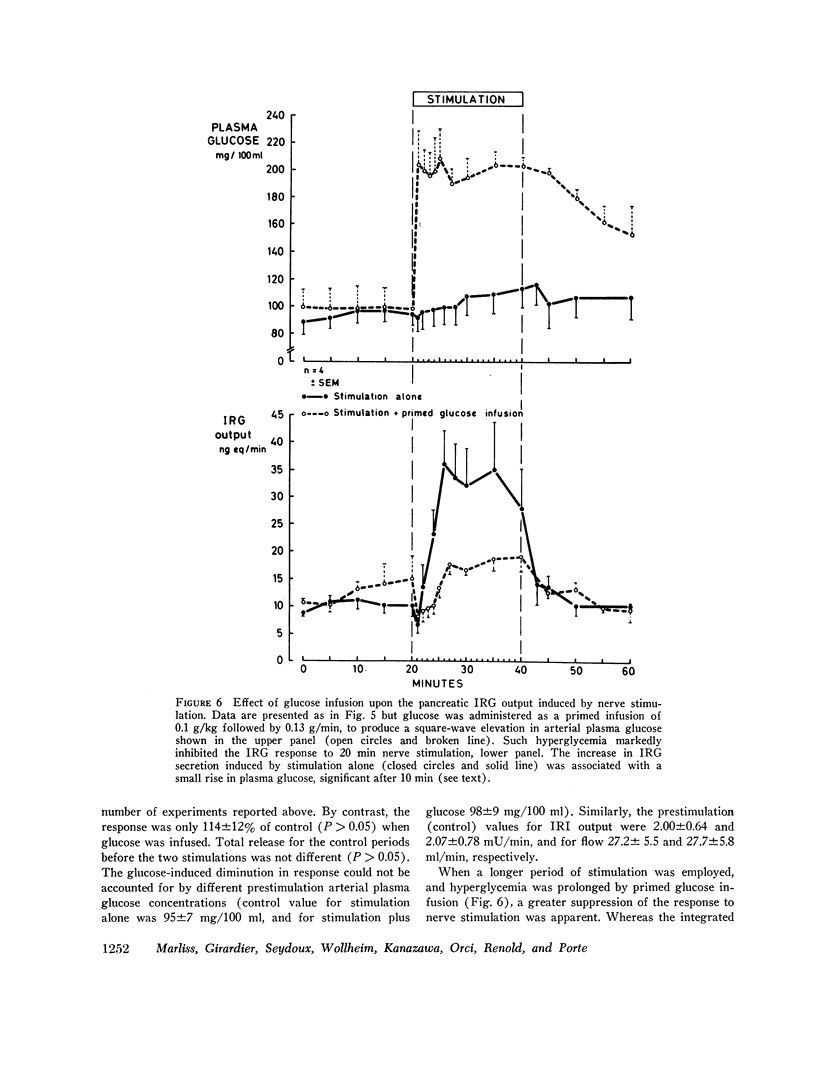
Electrical stimulation of the distal end of the discrete bundles of nerve fibers isolated along the superior pancreaticoduodenal artery was invariably followed by an increase in IRG output. With 10-min periods of nerve stimulation, the integrated response showed that the higher the control glucagon output, the greater was the increment. Atropinization did not influence the response to stimulation. That the preparation behaved in physiologic fashion was confirmed by a fall in IRG output, and a rise in immunoreactive insulin (IRI) output, during hyperglycemia induced by intravenous glucose (0.1 g/kg). The kinetics of this glucose effect on IRG showed characteristics opposite to those of nerve stimulation: the lower the control output, the less the decrement. Furthermore, during the control steady state, blood glucose concentration was tightly correlated with the IRI/IRG molar output ratio, the function relating the two parameters being markedly nonlinear. Injection or primed infusion of glucose diminished the IRG response to simultaneous nerve stimulation.
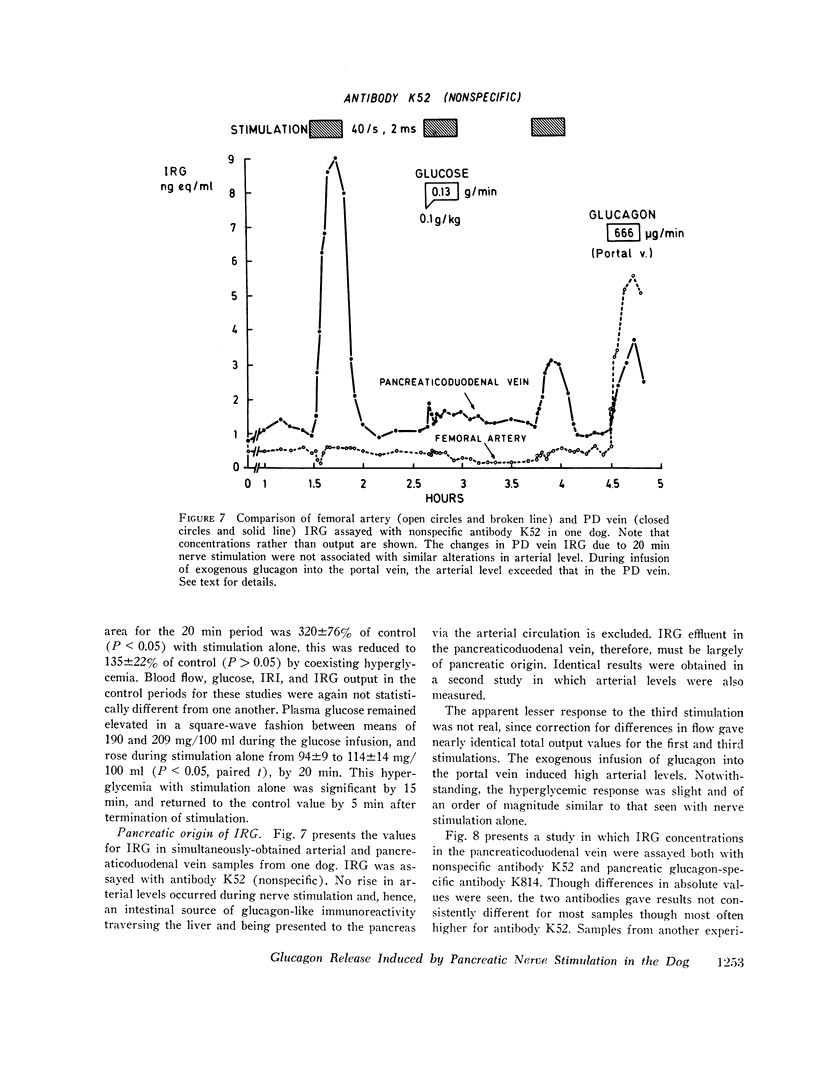
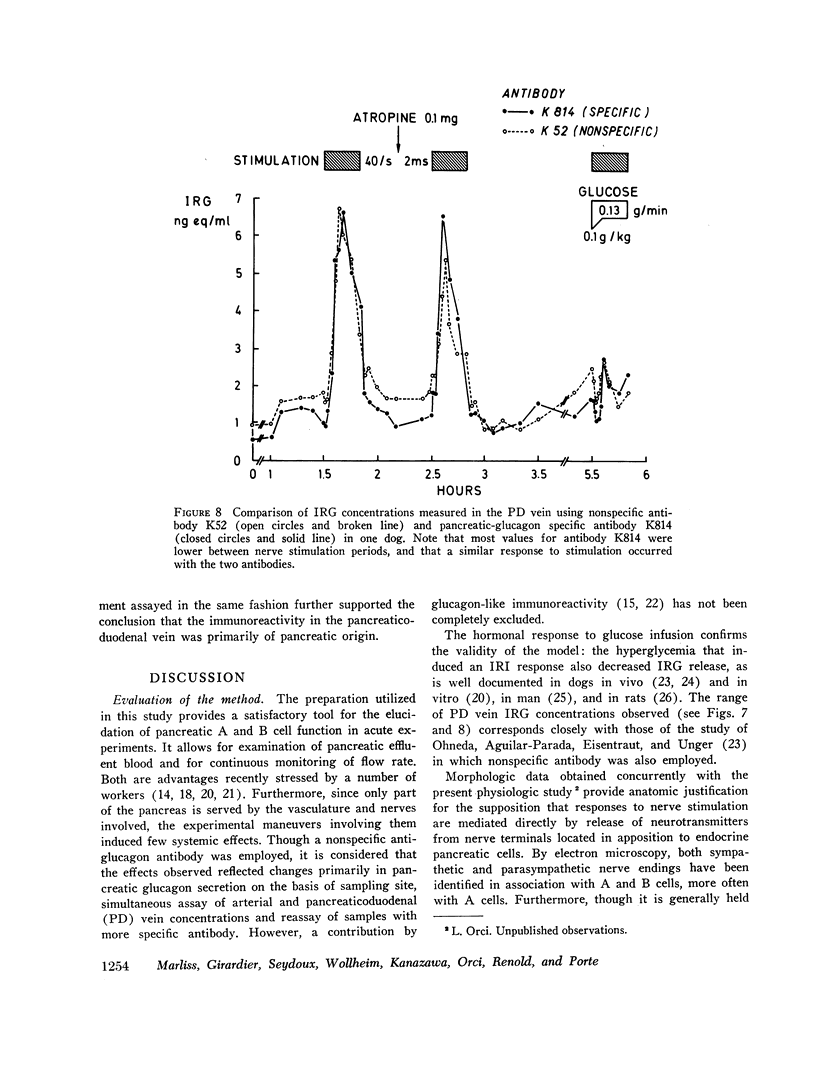
Measurement of IRG was inferred to reflect response of pancreatic glucagon secretion on the basis of the site of sample collection (the superior pancreaticoduodenal vein), the absence of changes in arterial IRG, and similar responses being obtained using an antibody specific for pancreatic glucagon.
These studies support a role for the autonomic nervous system in the control of glucagon secretion: direct nerve stimulation induces glucagon release. Such sympathetic activation may be interpreted as capable of shifting the sensitivity of the A cell to glucose in the direction of higher glycemia for a given glucagon output. The experimental model employed is valid for further studies of regulatory mechanisms of endocrine pancreatic function in vivo.
Full text
PDF


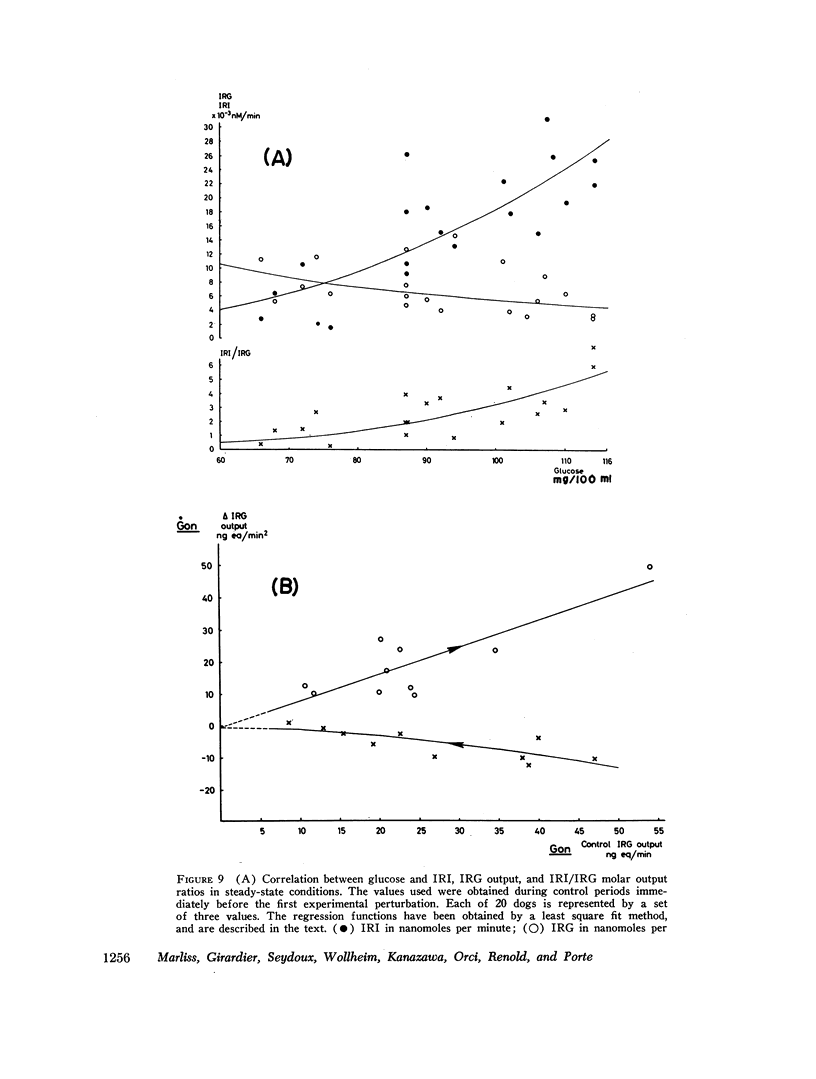



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assan R., Hautecouverture G., Guillemant S., Dauchy F., Protin P., Derot M. Evolution de paramètres hormonaux (glucagon, cortisol, hormone somatotrope) et énergétiques (glucose, acides gras, glycérol libre) dans dix acido-cétoses diabétiques graves traitées. Pathol Biol (Paris) 1969 Dec;17(23):1095–1105. [PubMed] [Google Scholar]
- BENCOSME S. A. Studies on the terminal autonomic nervous system with special reference to the pancreatic islets. Lab Invest. 1959 May-Jun;8(3):629–646. [PubMed] [Google Scholar]
- Baum D., Dillard D. H., Porte D., Jr Inhibition of insulin release in infants undergoing deep hypothermic cardiovascular surgery. N Engl J Med. 1968 Dec 12;279(24):1309–1314. doi: 10.1056/NEJM196812122792404. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970 May;19(5):302–306. doi: 10.2337/diab.19.5.302. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., Vance J. E., Dinstl K., Williams R. H. Effect of blood glucose on glucagon secretion in anesthetized dogs. Diabetes. 1969 Jan;18(1):11–18. doi: 10.2337/diab.18.1.11. [DOI] [PubMed] [Google Scholar]
- Burr I. M., Taft H. P., Stauffacher W., Renold A. E. On the role of cyclic AMP in insulin release: II. Dynamic aspects and relations to adrenergic receptors in the perfused pancreas of adult rats. Ann N Y Acad Sci. 1971 Dec 30;185:245–262. doi: 10.1111/j.1749-6632.1971.tb45253.x. [DOI] [PubMed] [Google Scholar]
- Böttger I., Schlein E. M., Faloona G. R., Knochel J. P., Unger R. H. The effect of exercise on glucagon secretion. J Clin Endocrinol Metab. 1972 Jul;35(1):117–125. doi: 10.1210/jcem-35-1-117. [DOI] [PubMed] [Google Scholar]
- Esterhuizen A. C., Howell S. L. Ultrastructure of the A-cells of cat islets of Langerhans following sympathetic stimulation of glucagon secretion. J Cell Biol. 1970 Sep;46(3):593–598. doi: 10.1083/jcb.46.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterhuizen A. C., Spriggs T. L., Lever J. D. Nature of islet-cell innervation in the cat pancreas. Diabetes. 1968 Jan;17(1):33–36. doi: 10.2337/diab.17.1.33. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Amino acid metabolism in exercising man. J Clin Invest. 1971 Dec;50(12):2703–2714. doi: 10.1172/JCI106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R., Ahlborg G. Plasma glucagon levels in exercising man. N Engl J Med. 1972 Jul 27;287(4):184–185. doi: 10.1056/NEJM197207272870412. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Bernardis L. L. Effect of hypothalamic stimulation on plasma glucose, insulin, and glucagon levels. Am J Physiol. 1971 Dec;221(6):1596–1603. doi: 10.1152/ajplegacy.1971.221.6.1596. [DOI] [PubMed] [Google Scholar]
- Girard J., Bal D., Assan R. Glucagon secretion during the early postnatal period in the rat. Horm Metab Res. 1972 May;4(3):168–170. doi: 10.1055/s-0028-1094093. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of pancreatic and gut glucagon in plasma. Diabetologia. 1971 Feb;7(1):10–19. doi: 10.1007/BF02346248. [DOI] [PubMed] [Google Scholar]
- Hinton P., Allison S. P., Littlejohn S., Lloyd J. Insulin and glucose to reduce catabolic response to injury in burned patients. Lancet. 1971 Apr 17;1(7703):767–769. doi: 10.1016/s0140-6736(71)91213-x. [DOI] [PubMed] [Google Scholar]
- Iversen J. Secretion of glucagon from the isolated, perfused canine pancreas. J Clin Invest. 1971 Oct;50(10):2123–2136. doi: 10.1172/JCI106706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen K. H., Larsen U. D. Purification of 125 I-glucagon by anion exchange chromatography. Horm Metab Res. 1972 May;4(3):223–224. doi: 10.1055/s-0028-1097092. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y., Kuzuya T., Ide T. Insulin output via the pancreatic vein and plasma insulin response to glucose in dogs. Am J Physiol. 1968 Sep;215(3):620–626. doi: 10.1152/ajplegacy.1968.215.3.620. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Fujita T. Fine structure of mammalian and avian pancreatic islets with special reference to D cells and nervous elements. Z Zellforsch Mikrosk Anat. 1969 Sep 17;100(3):340–363. doi: 10.1007/BF00571491. [DOI] [PubMed] [Google Scholar]
- Leclercq-Meyer V., Brisson G. R., Malaisse W. J. Effect of adrenaline and glucose on release of glucagon and insulin in vitro. Nat New Biol. 1971 Jun 23;231(25):248–249. doi: 10.1038/newbio231248a0. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S., Federspil G. Muscular exercise and pancreatic function in rats. Isr J Med Sci. 1972 Mar;8(3):390–398. [PubMed] [Google Scholar]
- Lefèbvre P., Luyckx A., Robaye B. Pattern of twenty-four plasma amino acids in rats before and after muscular exercise. Arch Int Physiol Biochim. 1972 Dec;80(5):935–940. doi: 10.3109/13813457209070443. [DOI] [PubMed] [Google Scholar]
- Legg P. G. The fine structure and innervation of the beta and delta cells in the islet of Langerhans of the cat. Z Zellforsch Mikrosk Anat. 1967;80(3):307–321. doi: 10.1007/BF00339324. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of alanine on glucagon secretion. J Clin Invest. 1971 Oct;50(10):2215–2218. doi: 10.1172/JCI106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda A., Aguilar-Parada E., Eisentraut A. M., Unger R. H. Control of pancreatic glucagon secretion by glucose. Diabetes. 1969 Jan;18(1):1–10. doi: 10.2337/diab.18.1.1. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Bagdade J. D. Human insulin secretion: as integrated approach. Annu Rev Med. 1970;21:219–240. doi: 10.1146/annurev.me.21.020170.001251. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Girardier L., Seydoux J., Kanazawa Y., Posternak J. Neural regulation of insulin secretion in the dog. J Clin Invest. 1973 Jan;52(1):210–214. doi: 10.1172/JCI107168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAHL M. ELEKTRONENMIKROSKOPISCHE UNTERSUCHUNGEN UEBER DIE VEGETATIVE INNERVATION DER BAUCHSPEICHELDRUESE. Z Mikrosk Anat Forsch. 1963;70:62–102. [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes. 1971 Dec;20(12):834–838. doi: 10.2337/diab.20.12.834. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Glucagon physiology and pathophysiology. N Engl J Med. 1971 Aug 19;285(8):443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]
- VENDSALU A. Studies on adrenaline and noradrenaline in human plasma. Acta Physiol Scand Suppl. 1960;49(173):1–123. [PubMed] [Google Scholar]
- Valverde I., Rigopoulou D., Marco J., Faloona G. R., Unger R. H. Characterization of glucagon-like immunoreactivity (GLI). Diabetes. 1970 Sep;19(9):614–623. doi: 10.2337/diab.19.9.614. [DOI] [PubMed] [Google Scholar]
- WINBORN W. B. LIGHT AND ELECTRON MICROSCOPY OF THE ISLETS OF LANGERHANS OF THE SAIMIRI MONKEY PANCREAS. Anat Rec. 1963 Sep;147:65–93. doi: 10.1002/ar.1091470107. [DOI] [PubMed] [Google Scholar]
- Watari N. Fine structure of nervous elements in the pancreas of some vertebrates. Z Zellforsch Mikrosk Anat. 1968;85(3):291–314. doi: 10.1007/BF00328843. [DOI] [PubMed] [Google Scholar]