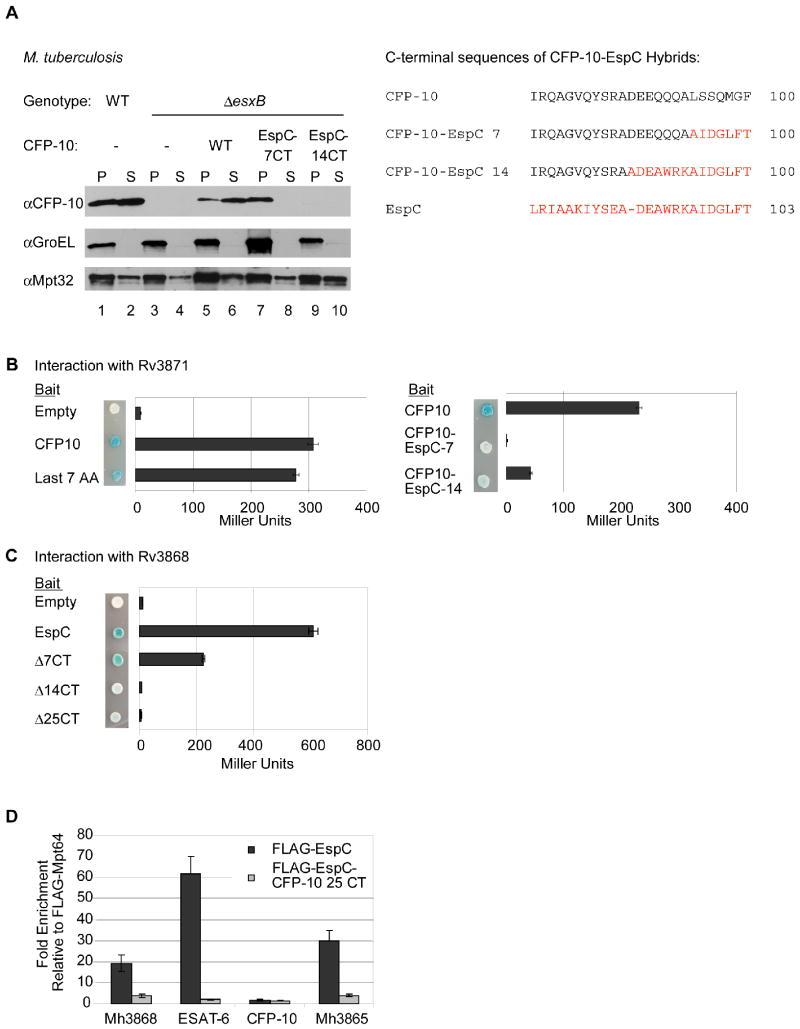
Fig. 2. Rv3868 interacts with the C-terminus of EspC in vitro and in vivo.
(A) Immunoblot analysis of cell pellets (P) and supernatants (S) from wild-type or ΔesxB M. tuberculosis cells harboring plasmids expressing ESAT-6 and either CFP-10 or CFP-10-EspC hybrid proteins. GroEL is used as a control for autolysis, and Mpt32 is used as a loading control for pellet and supernatant samples. The resulting C-terminal sequences of the CFP-10 EspC hybrid proteins are shown (right). (B) Yeast two-hybrid analysis of the interaction between CFP-10 or CFP-10-EspC hybrid proteins with Rv3871 (amino acids 248-591). (C) Yeast two-hybrid analysis of interaction between C-terminal truncations of EspC and Rv3868. For B and C, blue yeast colonies grown on solid media containing X-gal were positive for interaction. β-galactosidase activity is shown; error bars represent standard deviation. For all yeast two-hybrid experiments, M. tuberculosis constructs were used to study interaction. (D) Relative fold-enrichment of each protein selectively identified in the immunoprecipitation from M. marinum was assessed using quantitative mass spectrometry. The average enrichment over three injections is plotted for each protein. The fold enrichment was normalized to the average enrichment from immunoprecipitation with FLAG-Mpt64.