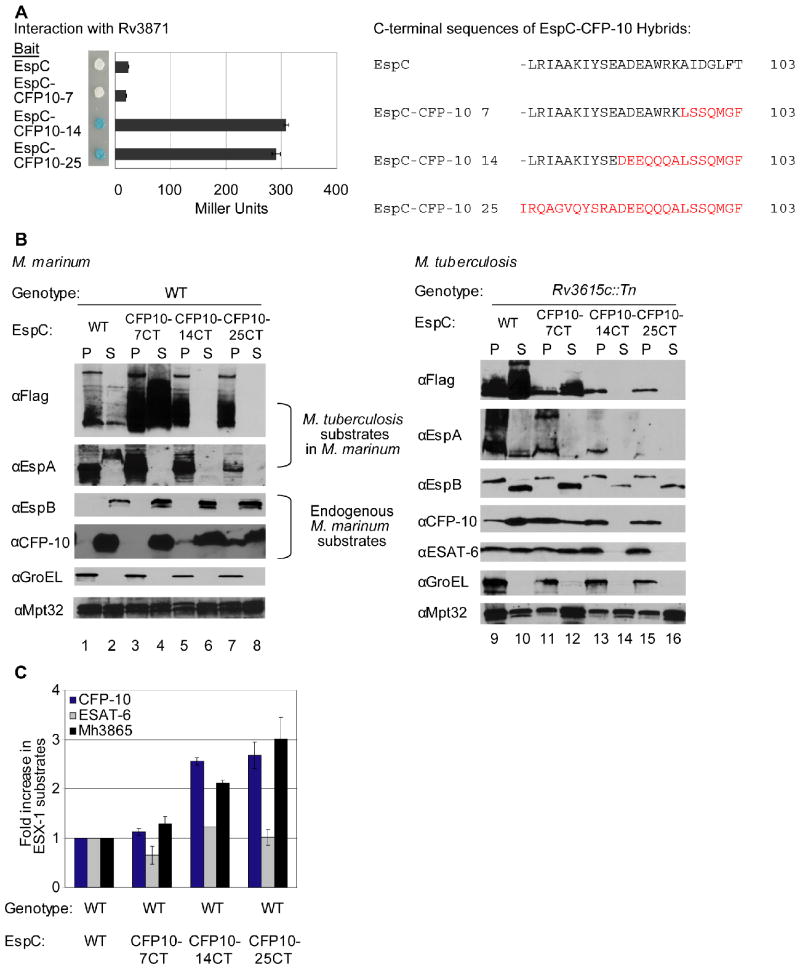
Fig. 3. Interaction of substrates with at least two ESX-1 associated ATPases is required for secretion.
(A) Yeast two-hybrid analysis of the interaction between EspC or EspC-CFP-10 hybrid proteins with Rv3868. Blue yeast colonies grown on solid media containing X-gal were positive for interaction. β-galactosidase activity is shown; error bars represent standard deviation. M. tuberculosis constructs were used to study interaction. The resulting C-terminal sequences of the EspC-CFP-10 hybrid proteins are shown (right). (B) Immunoblot analysis of cell pellets (P) and supernatants (S) of wild-type M. marinum strains (left) or M. tuberculosis bearing a transposon insertion in Rv3615c (right) expressing FLAG-EspC or FLAG-EspC-CFP-10 hybrid proteins. GroEL is used as a control for autolysis, and Mpt32 is used as a loading control for pellet and supernatant samples (C) Accumulation of M. marinum ESX-1 substrates in M. marinum due to expression of FLAG-EspC-CFP-10 hybrid proteins. Shown is the fold-change of cytosolic ESX-1 proteins in strains expressing the FLAG-EspC with the CFP-10 targeting region relative to wild-type M. marinum. 3 peptides were averaged together for CFP-10 and ESAT-6, and 5 were used for Mh3865 (EspFMm). Error bars are the %CV for the average of three measurements per peptide. Peak areas are available in the supplementary data (Table S4) and confirmatory spectra can be found in Figures S6 and S7.