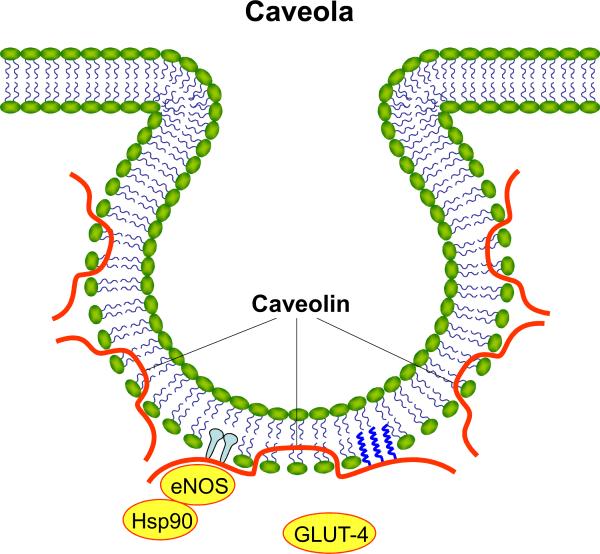
Volatile anesthetics produce important cardioprotective effects by stimulating a series of intracellular signaling events that ultimately render myocardium resistant to infarction. Anesthetics are known to protect the heart in a temporal manner. An initial early window of myocardial protection lasts hours after exposure to isoflurane or other volatile agents, and myocardial protection reappears again 24 to 48 h later. The mechanisms of early and delayed anesthetic preconditioning differ. Anesthetics activate various intracellular kinases which phosphorylate and subsequently modify the activity of downstream proteins (e.g., endothelial nitric oxide synthase [eNOS] and adenosine triphosphate-regulated potassium channels) that are important in mediating cardioprotection. During the early preconditioning phase, modification of preexisting proteins leads to protection, whereas after 24 h, cardioprotection relies on the synthesis of new proteins. The complexity of these signal transduction events requires both functional and spatial organization, and coordination of the activity of a large number of intracellular proteins. In this issue of the Journal, Tsutsumi et al1 demonstrate that isoflurane produces delayed protection against myocardial infarction by modulating a key protein, caveolin-3, found in membrane (lipid) rafts (fig. 1).
An extension of the classical fluid lipid bilayer model of the plasma membrane, lipid/membrane rafts are small (10–200 nm) microdomains enriched in sterols, sphingolipids and cholesterol “floating” in a sea of phospholipids.2 These lipid domains form docking platforms that control the location of intracellular signal transduction events. Rafts are located in the plasma membrane, and are also found in the endoplasmic reticulum and mitochondria. Membrane rafts function to regulate cellular processes by concentrating proteins to highly specific intracellular locations. The formation of lipid rafts is highly dynamic and this property allows for temporal regulation of protein signaling and trafficking. A subclass of membrane rafts are the caveolae, which are flask-like invaginations of the cellular membrane (60–80 nm), distinguished by the presence of scaffolding proteins caveolin-1, -2, and -3.3 Caveolins-1 and -2 are highly expressed in adipocytes, endothelial cells, and fibroblasts, whereas, caveolin-3 is expressed predominantly in skeletal, cardiac, and smooth muscle cells. Caveolae are disrupted in caveolin-1 and caveolin-3 knock out mice, but are preserved in caveolin-2 mutants.4 Caveolins bind proteins through a specific domain that enables conformational changes to occur and this action regulates the activity of signal transduction molecules. Caveolins are required for caveolae formation and their expression indirectly regulates the number of caveolae available for functional signal transduction. Caveolins can alter the fluidity of membrane rafts through the binding of cholesterol which in turn alters membrane composition and signaling effects.4 Tsutsumi et al5 have previously shown that cardiac-specific over-expression of caveolin-3 decreases myocardial infarction and mimics ischemic preconditioning. The current results extend these previous findings and demonstrate that isoflurane produces delayed preconditioning by up-regulating caveolin-3 and by increasing the co-localization of caveolin-3 with glucose-transporter (GLUT)-4 in membrane rafts (caveolar fraction).
GLUT-4 is the major transporter responsible for glucose uptake into cells. During ischemia, cardiac myocyte metabolism is altered to favor anaerobic glycolysis and increased GLUT-4 translocation from intracellular compartments to the plasma membrane facilitates substrate availability. GLUT-4 has previously been implicated in the cardioprotective effect of both early and delayed forms of ischemic preconditioning, a phenomenon in which brief periods of myocardial ischemia up to 2 (early) or 24–48 h (delayed) before a prolonged period of coronary artery occlusion and reperfusion decreases the extent of subsequent infarction. Myocardial ischemia appears to increase GLUT-4 expression and translocation. GLUT-4 protein is up-regulated after ischemic preconditioning along with increased expression of caveolin-3, phosphorylated eNOS, and phosphorylated Akt. Preconditioning stimuli not only increase the expression of these cardioprotective proteins, but also stimulate translocation of GLUT-4 to the caveolar-rich membrane fraction, thereby, sustaining activation of signaling molecules.6 Interestingly eNOS, which is known to play an important role during both early and delayed phases of anesthetic preconditioning, is reciprocally regulated by interactions with caveolins-1 and -3, and GLUT-4. The activity of eNOS is decreased when this enzyme is associated with caveolin-1. Conversely, disassociation of caveolin-1/eNOS interaction, and increased translocation of GLUT-4 and its enhanced association with caveolin-3 activates eNOS.7 Although caveolin-1 is an important mediator of the early phase of isoflurane preconditioning, there appears to be almost no role for this protein in delayed protection. These findings indicate a differential role of caveolins during anesthetic cardioprotection that may be both temporal (early vs. delayed) and cell lineage (endothelial cell vs. cardiomyocyte) specific. Caveolin-1, while negatively regulating eNOS under basal conditions, also facilitates eNOS signaling during stimulation by compartmentalizing proteins in the appropriate intracellular locations. This condition is referred to as “caveolar paradox” and illustrates the complex nature of protein-protein interactions that ultimately impacts cell survival after ischemia and reperfusion.
Other binding proteins, such as heat shock protein-90, have also been implicated in the regulation of eNOS by caveolins. For example, caveolin-1, eNOS, and heat shock protein 90 can be co-immunoprecipitated from endothelial cells, and the presence of heat shock protein 90 decreases the inhibitory effect of caveolin-1 on eNOS activity.8 Tsutsumi et al1 did not investigate eNOS regulation by GLUT-4/caveolin-3 interactions during delayed anesthetic preconditioning; however, it is interesting to speculate that interactions between these molecules and other binding partners, such as heat shock protein 90, may be spatially regulated in caveolae by lipophilic volatile anesthetics.
GLUT-4 is the major insulin-responsive glucose transporter. Insulin-induced translocation of GLUT-4 to the membrane from intracellular vesicles stimulates glucose uptake in muscle and adipose tissues. However, the exact mechanism whereby translocation of GLUT-4 to the plasma membrane induces cardioprotection is unknown. The current findings that GLUT-4 translocation is enhanced by delayed preconditioning with isoflurane in caveolin-1 but not -3 knockout mice also implicates caveolins as potential targets of disease processes, such as diabetes, that modulate the efficacy of preconditioning. For example, diabetes and acute hyperglycemia attenuate reduction of myocardial infarct size elicited by diverse preconditioning stimuli and this occurs through impaired eNOS regulation by heat shock protein 90.9 The caveolins are also modulated by diabetes. Caveolin-3 content is reduced in lipid rafts from diabetic myocardium7 and disruption of caveolae in adipocytes renders these cells insulin resistant.10 Caveolin-3 knock-out mice exhibit insulin resistance in vivo, and acute hyperglycemia alone disturbs lipid raft stability by interfering with cholesterol synthesis.11 Thus, defects in membrane raft composition and caveolin expression induced by disease states could underlie the lack of cardioprotection observed in some clinical studies using volatile anesthetics, although, this hypothesis remains to be specifically tested.
The work by Tsutsumi and colleagues1 highlighted in this issue of the Journal emphasizes that anesthetic cardioprotection requires functional lipid domains, such as caveolae, and their associated proteins the caveolins. Membrane rafts regulate the intracellular location of signaling molecules activated by volatile anesthetics and these microdomains may be the interface through which anesthetics directly mediate protection. Moreover, lipid rafts may represent a new target for the rescue of ischemic myocardium and provide a new therapeutic approach in the treatment of patients with cardiovascular disease.
Footnotes
The authors are not supported by, nor maintain any financial interest in, any commercial activity that may be associated with the topic of this article
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tsutsumi YM, Kawaraguchi Y, Horikawa YT, Niesman IR, Kidd MW, Chin-Lee B, Head BP, Patel PM, Roth DM, Patel HH. Role for caveolin-3 and glucose transporter 4 in isoflurane-induced delayed cardiac protection. Anesthesiology. 2010;112:XXXXXX. doi: 10.1097/ALN.0b013e3181d3d624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pike LJ. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–8. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AW, Combs TP, Scherer PE, Lisanti MP. Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1151–60. doi: 10.1152/ajpendo.00324.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–17. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation. 2008;118:1979–88. doi: 10.1161/CIRCULATIONAHA.108.788331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koneru S, Penumathsa SV, Thrirunavukkarasu M, Samuel SM, Zhan L, Han Z, Das DK, Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–72. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 7.Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Maulik G, Bagchi M, Bagchi D, Maulik N. Niacin bound chromium treatment induces myocardial Glut-4 translocation and caveolar interaction via Akt, AMPK and eNOS phosphorylation in streptozotocin induced diabetic rats after ischemia-reperfusion injury. Biochim Biophys Acta. 2009;1792:39–48. doi: 10.1016/j.bbadis.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–72. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 9.Amour J, Brzezinska AK, Jager Z, Sullivan C, Weihrauch D, Du J, Vladic N, Shi Y, Warltier DC, Pratt PF, Kersten JR. Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms. Anesthesiology. 2010;112:XXXXX. doi: 10.1097/ALN.0b013e3181cded1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem. 2001;276:9670–8. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- 11.Somanath S, Barg S, Marshall C, Silwood CJ, Turner MD. High extracellular glucose inhibits exocytosis through disruption of syntaxin 1A-containing lipid rafts. Biochem Biophys Res Commun. 2009;389:241–6. doi: 10.1016/j.bbrc.2009.08.126. [DOI] [PubMed] [Google Scholar]