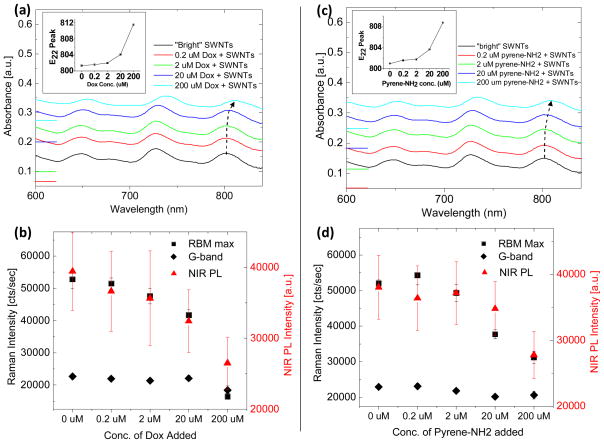
Figure 5.
Non-covalent interaction of small aromatic molecules with DGC separated SWNTs causes similar effects as SWNT bundling. The loading of Doxorubicin (Dox) onto DGC separated, “bright” SWNTs causes a (a) concentration-dependent red-shifting of SWNT optical transition absorption peaks. Absorption spectra are offset vertically for clarity and zeroes are marked on the left axis. The peak position of the optical transition near 800 nm is plotted in the inset. (b) The intensity of the RBM at 233 cm−1 (black squares) as well as that of the G-band at 1590 cm−1 (black diamonds) decrease with increasing Dox concentration and subsequent optical transition red-shift. An accompanying decrease in photoluminescence intensity is also observed (red triangles). The loading of 1-pyrenemethylamine (pyrene-NH2) onto DGC-separated, “bright” SWNTs causes a similar, but slightly weaker effect, on (c) the SWNT optical transitions and (d) Raman scattering and photoluminescence intensities.