Abstract
The purpose of this work was to determine in colon mucosa of Crohn’s disease (CD) and ulcerative colitis (UC) in relapse: a) the levels of the chaperonins Hsp60 and Hsp10; b) the quantity of inflammatory cells; and c) if the levels of chaperonins parallel those of inflammation cells. Twenty cases of CD and UC and twenty normal controls (NC) were studied using immunohistochemistry, Western blotting and immunofluorescence. Immunohistochemically, Hsp60 and Hsp10 were increased in both inflammatory bowel diseases (IBD) compared to NC. These results were confirmed by Western blotting. Hsp60 and Hsp10 occurred in the cytoplasm of epithelial cells in CD and UC but not in NC. Hsp60 and Hsp10 co-localised to epithelial cells of mucosal glands but not always in connective tissue cells of lamina propria, where only Hsp60 or, less often, Hsp10 was found. Cells typical of inflammation were significantly more abundant in CD and UC than in NC. Since chaperonins are key factors in the activation of the immune system leading to inflammation, we propose that they play a central role in the pathogenesis of the two diseases, which, consequently, ought to be studied as chaperonopathies.
Keywords: Chaperonins, Inflammation, IBD, Chaperonopathies, Autoimmunity
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are serious pathologies whose etiology and pathogenesis are still incompletely understood (Bouma and Strober 2003; Kucharzik et al. 2006; Devlin and Panaccione 2010). They are considered inflammatory bowel diseases (IBD) because inflammation of the gastrointestinal tract is a major component (Fukushima et al. 1991; Bouma and Strober 2003; Hibi and Ogata 2006; McGuckin et al. 2009; Puga Yung et al. 2009). However, why and how inflammation develops and is maintained through periods of relapse and remission are unanswered questions. Modern chaperonology has taught us that Hsp-chaperones such as Hsp70, Hsp60 and Hsp10 play what appears to be a critical role in the inflammatory response to a number of noxae or stressors and in immune regulation (Ludwig et al. 1999; Wang et al. 2002, 2005; Imamura et al. 2005; Johnson et al. 2005; Corrao et al. 2010; Macario et al. 2010). It could be, therefore, pertinent to revisit CD and UC under the light of this new information in order to determine whether Hsp-chaperones are involved in these diseases and what is their role in pathogenesis. We focused on the chaperonins Hsp60 and Hsp10. Both were traditionally considered intracellular molecules, confined to the mitochondria and dedicated to assist in protein folding. However, in the last several years Hsp60 and Hsp10 have been found in other locations, outside the mitochondria, such as cytosol, cell membrane, intercellular space, and in blood (Pockley and Multhoff 2007; Pockley et al. 2007; Cappello et al. 2008; Corrao et al. 2010). Moreover, both chaperonins increased during carcinogenesis of a number of anatomical regions (Cappello et al. 2002, 2003a, b) including the large bowel (Cappello et al. 2003b, c, 2005). Finally, it has also been established that during organ pathogenesis both chaperonins, particularly Hsp60, play roles unrelated to protein folding, one of which is interaction with, and activation of, cells involved in the innate and adaptive immune responses (Kol et al. 2000; Ohashi et al. 2000; Johnson et al. 2005; Kamphuis et al. 2005; Imamura et al. 2005; Osterloh et al. 2007, 2008; Nara et al. 2008; Henderson et al. 2010; Macario et al. 2010).
As a first step toward determining whether or not Hsp60 and Hsp10 are involved in the pathogenesis of inflammation in CD and UC we decided to investigate their levels in biopsy specimens from a group of well studied cases. The participation of chaperones in the pathogenesis of autoimmune diseases, including IBD, has been postulated before (Stevens et al. 1992; Ludwig et al. 1999; Stahl et al. 1998; Sukegawa et al. 2000; Imamura et al. 2005; Kamphuis et al. 2005; Nara et al. 2008; Puga Yung et al. 2009) but information on Hsp60 and Hsp10 localization and levels in mucosal samples from CD and UC patients is scarce, or lacking in the case of Hsp10. The present work aims at filling this gap and, thus, at elucidating whether or not Hsp60 and Hsp10 should be further investigated as possible disease biomarkers useful in diagnosis and prognosis, etiologic-pathogenetic factors, or therapeutic targets in IBD.
Materials and methods
Specimens
Specimens for study were archival tissues stored in the Department of Human Pathology, University of Palermo, Italy. The specimens were from 40 large bowel biopsies taken via colonoscopy from 40 patients in relapse, 20 CD and 20 UC cases diagnosed between 2006 and 2008. Informed consent was obtained from patients at the time of biopsy. Diagnosis of IBD and classification into CD and UC were based on standard clinical, endoscopic, histologic and radiologic information. Twenty specimens were similarly collected from 20 normal colons as controls. The age of all subjects ranged between 28 and 65 years. All the specimens were formalin-fixed and paraffin-embedded. From each case, 4-5 μm sections were obtained for immunohistochemical and immunofluorescence analyses, and thick sections (25 μm) for protein extraction to carry out western blotting.
Immunohistochemistry
Immunostaining was done with a battery of antibodies listed in Table 1 on 5-μm sections, using an avidin-biotin complex kit (LSAB2, DAKO, Carpinteria, CA, USA). Appropriate positive controls, as well as non-immune serum for negative controls, were run concurrently. 3-3'-diaminobenzidine (DAB chromogen solution, DAKO) was used as developer chromogen. Nuclear counterstaining was made using haematoxylin (DAKO).
Table 1.
Characteristic of the antibodies used in this study
| Antibody specific for: | Supplier | Catalogue number | Source | Dilution |
|---|---|---|---|---|
| Hsp60 | SIGMA | H4149 | mouse | 1:300 |
| Hsp10 | StressGen | SPA-110 | rabbit | 1:300 |
| CD3 | Novocastra | NCL-L-CD3-PS1 | mouse | 1:100 |
| CD4 | Novocastra | NCL-L-CD4-1F6 | mouse | 1:100 |
| CD8 | Novocastra | NCL-L-CD8-295 | mouse | 1:100 |
| CD20 | Novocastra | NCL-L-CD20-L26 | mouse | 1:100 |
| CD68 | Novocastra | NCL-CD68-KP1 | mouse | 1:100 |
Three independent observers (FC, AM and VR) examined the specimens in blind (code marked) and performed a semiquantitative analysis to evaluate: a) the percentage of epithelial cells positive for chaperonins (Hsp60 and Hsp10), and b) the percentage of stromal cells positive for leukocyte markers (CD3, CD4, CD8, CD20, and CD68). All the observations were made at a magnification of 200X and the means of the triplicate counts were used for statistical analyses.
Western blotting
Samples for western blotting were subjected to SDS-PAGE electrophoresis on, respectively, a 10% (for Hsp60) and a 15% (for Hsp10) polyacrylamide minigel (Bio-Rad Laboratories Inc, Milan, Italy). Total proteins were extracted from thick (25 μm) paraffin-embedded sections, following a standard protocol, (Ikeda et al. 1998) and were loaded at 30 µg/lane, alongside lanes with a molecular weight marker (Bio-RAD Laboratories, Italy). After electrophoresis, proteins were transferred to nitrocellulose membranes. After transfer, all membranes were stained with Poinceau S to verify loading consistency and transfer quality. The membranes were blocked with 5% fat milk, and then probed for 12 hours with the specific antibodies, overnight at 4°C. The primary antibodies used were mouse anti-Hsp60 and rabbit anti-Hsp10 (see Table 1 for details). Antibodies were diluted following the manufacturer’s instructions in antibody buffer (1% bovine serum albumin in 0.05% Tween-20 TBS). Antibody binding to blotted antigens was revealed by incubation with ECL detection reagents (Amersham Pharmacia Biotech, Uppsala, Sweden) and exposure to an autoradiographic film (Kodak BioMax, Sigma-Aldrich, Inc, Milan, Italy). The same membranes were stripped with a stripping buffer (Restore TM Western Blot Stripping Buffer, Pierce, Rockford, IL, USA) and incubated with the house-keeping protein beta-actin (Sigma-Aldrich, Inc, Milan, Italy) to normalize differences in protein loading if any, following the procedures described above. Densitometric analysis of blots was performed using the NIH Image J 1.40 analysis program (National Institutes of Health, Bethesda, MD, USA). Experiments were performed in triplicate. Results were normalized and expressed as the ratio of the quantification of the band intensity of protein tested after correction with the band intensity obtained for the beta-actin. Means of the triplicate measurements were used for statistical analyses.
Double immunofluorescence
After dewaxing in xylene, rehydration in ethanol and washing in phosphate buffer solution (PBS), 4-5 μm sections were incubated with unmasking solution (tri-sodium citrate 10 mM, 0.05% tween 20) for 10 min at 58°C and treated with blocking solution (3% albumin bovine serum in PBS) for 30 min at 24°C. Then, the sections were incubated with the first primary antibody (mouse anti-Hsp60, see Table 1 for details) overnight at 4°C. The day after, some sections were incubated with the second primary antibody (rabbit anti-Hsp10, see Table 1 for details) overnight at 4°C. After washing two times in PBS all sections were incubated with fluorescent secondary antibodies: mouse IgG antibody conjugated with FITC (Sigma-Aldrich, Inc, Milan, Italy) and/or rabbit IgG antibody conjugated with Texas Red (Gene Tex Inc., Irvine, CA, USA) for 1 h each at 24°C. The nuclei were counterstained with Hoechst (Sigma-Aldrich, Inc, Milan, Italy) for 15 min at 24°C. Finally, the sections were covered with a drop of PBS, the slides were mounted with cover slips and readings and imaging were immediately performed with a Leica DM5000 upright fluorescence microscope (Leica Microsystems, Heidelberg, Germany).
Statistical analysis
Data obtained from the evaluation of the immunohistochemical reactivity, as well as from Western blotting densitometry, were plotted using Microsoft Excel software (Microsoft Italia, Milan, Italy). Statistical analyses were carried out using the GraphPad Prism 4.0 package (GraphPad Inc., San Diego, CA, USA) and non parametric tests to compare groups that did not fit to the normal distribution. To evaluate the significance of differences of both Hsp60 and Hsp10 levels between the CD and UC, the Mann-Whitney test was used. Correlation analysis between either Hsp60 or Hsp10 levels and inflammatory markers (CD3, CD4, CD8, CD20, and CD68) were performed using the non parametric Spearman’s test, which gives a correlation coefficient (Spearman r) and a “p” value that measures the significance of the analysed correlation. For correlation graphs, linear regression was also calculated to reflect the type of correlation between the chosen variables. Data were considered significant at an alpha-level of 5%. Finally, in order to have an indication on the variability of immunoreactivity data obtained by the three different observers, the coefficient of variation was calculated using both the inter-observer and intra-observers kappa statistics.
Results
The results displayed in Table 2 show that both Hsp60 and Hsp10 levels were higher in CD than in UC (p < 0.01) or normal colon (p < 0.005). Likewise, the levels of the two chaperonins were higher in UC than in normal colon (p < 0.01). Inter-observer and intra-observer kappa statistics for semiquantitative measurements of immunohistochemical reactions showed values of 0.85 and 0.95, respectively. The kappa statistic (or kappa coefficient) is the most commonly used statistic for measuring the agreement between two or more observers. It takes into account the fact that observers will sometimes agree or disagree simply by chance. A kappa of 1 indicates perfect agreement, whereas a kappa of 0 indicates agreement equivalent to chance (Viera and Garrett 2005). The calculation is based on the difference between how much agreement is actually present (“observed” agreement) compared to how much agreement would be expected to be present by chance alone (“expected” agreement). Our values of kappa statistic constitutes a “very good” agreement.
Table 2.
Presence of Hsp60 and Hsp10 in Crohn’s disease and ulcerative colitis in colon epithelial cells compared with frequency of CD-leukocyte markers in lamina propria
| Groups | Comparison between groupsb | |||||
|---|---|---|---|---|---|---|
| CDc | UC | NC | CD > NC | UC > NC | CD > UC | |
| Epithelial cells (percent) with:a | ||||||
| Hsp60 | 77 | 26 | 3 | p < .005 | p = .01 | p = .01 |
| Hsp10 | 84 | 43 | 5 | p = .005 | p = .01 | p = .01 |
| Lamina propria cells (percent) with the leukocyte marker: | ||||||
| CD3 | 19 | 16 | 8 | p < .005 | p < .01 | p > .05 |
| CD4 | 16 | 12 | 7 | p < .01 | p < .05 | p > .05 |
| CD8 | 14 | 13 | 5 | p < .01 | p < .01 | p > .05 |
| CD20 | 15 | 13 | 7 | p < .01 | p < .05 | p > .05 |
| CD68 | 16 | 17 | 8 | p < .01 | p < .01 | p > .05 |
aArithmetic means of triplicate counts. bStatistical methods applied are described under Materials and Methods. cAbbreviations are: CD, Crohn’s disease; UC, ulcerative colitis; NC, normal colon.
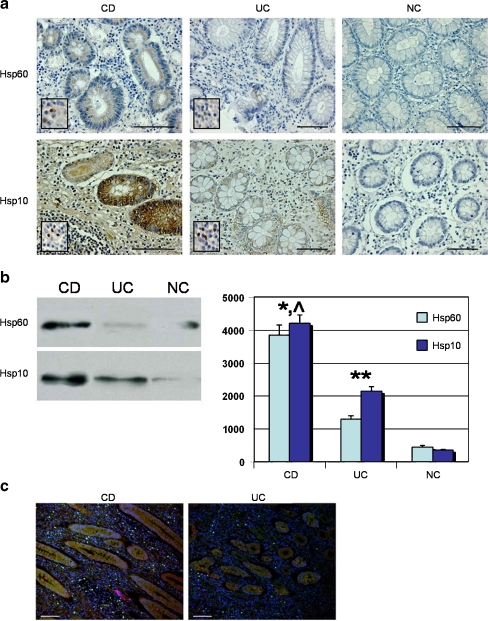
Representative immunohistochemical images are shown in Fig. 1a. Immunostaining for Hsp60 was as a rule less intense than that for Hsp10 and this is probably due to the higher avidity of the anti-Hsp10 antibody compared with the anti-Hsp60 antibody, as described previously (Cappello et al. 2003a). As it is apparent in the images, Hsp60 and Hsp10 were present in the cytoplasm of epithelial cells in CD and UC specimens, mainly in the abluminal region of the cells. Scattered leukocytes, fibrocytes, and endothelial cells in lamina propria also contained detectable levels of the two chaperonins (Fig. 1a, inserts). The chaperonins were scarcely present or undetectable in biopsies from normal colons, either in epithelium or lamina propria.
Fig. 1.
a) Illustrative images of the immunohistochemical demonstration of Hsp60 and Hsp10 in biopsy spcimens. Bar = 200 μm. Higher levels of both chaperonins are apparent in epithelial cells of both Crohn’s disease (CD) and ulcerative colitis (UC) by comparison with normal colon (NC), which appears slightly positive or negative. Epithelium is represented by round- or oval- shaped glands, among which lamina propria is interspersed. Inserts show chaperonin positivity in lamina propria cells (bar = 100 µm). b) Demonstration of Hsp60 and Hsp10 by Western blotting. Left panel. Representative Western blotting carried out for detecting Hsp60 and Hsp10 in total protein preparations from thick paraffin-embedded sections of CD, UC and NC biopsies. Right panel. The histogram represents the levels in arbitrary units (vertical axis, 0 to 5000) of Hsp60 and Hsp10 (as indicated by the distinctive column colors) as mean percentages +/- SD, determined in three separate experiments. The values were obtained with the NIH image J 1.40 analysis software. Hsp60 and Hsp10 in CD (*) and UC (**) are significantly higher (respectively p < 0.001 and p < 0.005) than in NC. Likewise, the levels of both chaperonins in CD (^) are significantly higher than in UC (p < 0.005). c) Double immunofluorescence reaction in colon biopsies. Hsp60 and Hsp10 colocalize in CD and UC specimens in the round- or oval- shaped mucosal glands (stained in orange). In contrast, lamina propria, interspersed among glands, showed frequently a single chaperonin, more often Hsp60 (green spots) than Hsp10 (red spots). Nuclei were counterstained in blue (bar = 100 µm)
Western blotting confirmed the immunohistochemical semiquantitative results (Fig. 1b). Double immunofluorescence showed that Hsp60 and Hsp10 co-localised to epithelial cells of mucosal glands (Fig. 1c) but not always in connective tissue cells of lamina propria, where often only one chaperonin was found, more frequently Hsp60 than Hsp10.
No appreciable differences were found between CD and UC for cells with the leukocyte markers investigated, CD3, CD4, CD8, CD20, and CD68. However, cells with these markers were more abundant in both CD and UC than in normal colons (Table 2). These results reflected the inflammatory reaction occurring in the patients’ colon mucosa and were in agreement with data from others (Fukushima et al. 1991; Rugtveit et al. 1994; Hibi and Ogata 2006).
A search for correlations between levels of Hsp60/Hsp10 and levels of cells positive to leukocyte markers (CD3, CD4, CD8, CD20, or CD68) did not produce any evidence for such correlations (data not shown).
Discussion
We found a consistent and significant increase in the chaperonins Hsp60 and Hsp10 in the colon mucosa of patients with CD and UC in comparison with normal colons, and the increase was more pronounced in CD than in UC. Increase of Hsp10 in these diseases is reported here for the first time. This contribution is important because Hsp10 is functionally associated with Hsp60, which has been studied before and whose variations should be correlated with those of its functional partner, Hsp10.
Our data on Hsp60 are in agreement with those reported previously, pertaining to 14 patients with CD and seven patients with UC (Peetermans et al. 1995). Particularly, the intestinal mucosa and submucosa of patients with CD and the mucosa of patients with UC showed strong Hsp60 immunopositivity, while only a few positive cells where found in controls. However, another study showed Hsp60 immunoreactivity in epithelial, vascular smooth and nerve cells in mucosal biopsies from patients with CD and also in controls (Baca-Estrada et al. 1994). These latter results differ from ours possibly because we focused on large bowel specimens from patients in relapse, while Baca-Estrada and co-workers examined both small and large bowel specimens without regard to disease stage.
In view of the data and the new developments pertaining to the biology and physiology of Hsp60 and Hsp10, particularly their role in immune system activation, we propose that a primary factor in colon pathogenesis of IBD is the augmentation of the two chaperonins in the mucosa on an inflammation platform. The elevated levels of the two chaperonins we consistently found in CD and UC could reflect one or more of various alternative physiopathological phenomena:
Non-disease specific reaction to mucosal stress and/or response to the colon tissue’s need for a higher rate of protein synthesis and, consequently, a pressing need of folding of new polypeptides in the face of protein destruction due to the pathological process linked to inflammation. In this situation, Hsp60 and Hsp10, which are supposed to assist in protein folding and refolding and translocation inside the mitochondria (Tatsuta and Langer 2008; Tatsuta 2009; Endo and Yamano 2009; Corrao et al. 2010) would be increased due to a response of the cell to a need not necessarily typical of CD or UC but that could occur in many other pathologies with inflammation and tissue destruction. In this case, elevated levels of Hsp60 and Hsp10 would reflect an anti-pathogenetic mechanism in which the two chaperonins would perform the canonical function of cytoprotection rather than contribute to the disease (Otani et al. 1997; Petrof et al. 2004; Cappello and Zummo 2005; Czarnecka et al. 2006; Otaka et al. 2006; Corrao et al. 2010). In line with this reasoning and from the practical standpoint, it may be stated that the elevated levels of the chaperonins could be used as indicators of disease and degree of defense against it and, thus, could have some value as diagnostic markers, and in assessing prognosis and response to treatment.
The elevated levels of Hsp60 and Hsp10 in diseased mucosa could be an indication that the two chaperonins are part of antigen-antibody complexes formed because they have become auto-antigens and are recognized by the patient’s immune system as foreign. This change from tolerance to immunity, could be due to: a) structural alterations suffered by the human chaperonins, such as pathological post-translational modifications due to oxidative stress or many other factors that can modify proteins, which make the human molecules immunogenic with regard to its own immune system (Macario et al. 2010); or b) cross reaction of the human Ab with a foreign Hsp, for example, from a bacterium colonized in the patient’s gastrointestinal or respiratory or genitourinary tracts or skin (van Eden 1991; van Eden et al. 2007; Cappello et al. 2009). In this scenario, antibodies are elicited by the foreign immunogen, which differs in some critical epitopes with regard to the patient’s equivalent molecule, and these antibodies cannot discriminate between the immunogen and the human molecule, which although normal and non-immunogenic (i.e., incapable of eliciting an immune response by itself) is recognized as an antigen by the antibodies to the foreign chaperonin. Since we now know that Hsp60 and Hsp10 travel everywhere outside cells it is possible for the immune system and antibodies to encounter these molecules, including the autologous ones, and react against them. Accumulation of antigen-antibody complexes in the mucosa would trigger a series of pathological events leading to perpetuation of inflammation and tissue destruction (Clynes et al. 1999; Shashidharamurthy et al. 2008; Mayadas et al. 2009). In this situation the two chaperonins would have a pathogenic effect, opposite to the cytoprotection mentioned in the alternative (1) describe above, namely a pathogenic effect typical of autoimmune diseases. From the practical viewpoint, measurement of anti-chaperonin antibodies would be a diagnostic tool and blockage of the immune system would be a therapeutic strategy for consideration.
A third alternative that might explain the elevated tissue levels of Hsp60 and Hsp10 derives from recent knowledge that these two proteins, particularly Hsp60, reside in the extracellular space and circulate in the blood and other body fluids, and can interact with components of the immune system everywhere (Corrao et al. 2010; Macario et al. 2010; Merendino et al. 2010). As a result of this interaction, cells of the innate immune system such as macrophage-monocytes and their equivalents in all tissues, dendritic cells, and neutrophils, are activated to perform pro-inflammatory functions linked to cyto- and chemokine production and release. The chaperonins can also activate immune cells pertaining to adaptive immune responses. In this regard, it is pertinent to mention that Hsp60-derived peptides were found to stimulate an inflammatory response accompanied by production of pro-inflammatory cytokines in intestinal mucosa of patients with CD (Puga Yung et al. 2009). Consequently, it could very well be that Hsp60, and perhaps also Hsp10, act as activators of pathogenic mechanisms mediated by the immune system.
In summary, considering the preceding alternatives 2 and 3, the primary culprit in CD and UC would be Hsp-chaperones, particularly Hsp60 and Hsp10, and the two diseases could be regarded as chaperonopathies (Macario and Conway de Macario 2005, 2007; Macario et al. 2010). The chaperonin molecules could be structurally intact but quantitatively increased due to gene dysregulation, in which case CD and UC would be dysregulatory chaperonopathies (Macario and Conway de Macario 2005, 2007).
It is pertinent to mention here that the human hsp60 and hsp10 genes are localised near each other, head-to-head on the same chromosome and are separated by a single bidirectional promoter (Hansen et al. 2003). This locus organization lends itself to co-regulation of the two genes with simultaneous overexpression, for example, in response to inducers such as biological and chemical stressors. Dysregulation of the locus could be linked to genetics, a factor known to play a role in IBD, and that could also, or instead, determine abnormal response of the immune system cells to interaction with the chaperonins. Alternatively, the chaperonin molecules could be structurally changed due to post-translational modifications for example, in which case the two diseases ought to be classified as acquired structural chaperonopathies. If the human chaperonins act as autoantigens or activate immune system cells in a pathologic manner the two diseases could be considered a subtype of the chaperonopathies by mistake (Macario and Conway de Macario 2007). These distinctions are important because treatment modality will be dictated by the type of pathology.
Acknowledgements
This work was supported by funds of University of Palermo (VR, SD, GZ and FC) and Istituto Euro-Mediterraneo di Scienza e Tecnologia (FC).
Footnotes
Vito Rodolico and Giovanni Tomasello contributed equally to the present work.
References
- Baca-Estrada ME, Gupta RS, Stead RH, Croitoru K. Intestinal expression and cellular immune responses to human heat-shock protein 60 in Crohn’s disease. Dig Dis Sci. 1994;39:498–506. doi: 10.1007/BF02088334. [DOI] [PubMed] [Google Scholar]
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Cappello F, Zummo G. HSP60 expression during carcinogenesis: a molecular “proteus” of carcinogenesis? Cell Stress Chaperones. 2005;10:263–264. doi: 10.1379/1466-1268(2005)10[263:HEDCAM]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, Palma A, Marciano V, Martorana G, Belfiore P, Martorana A, Farina F, Zummo G, Bucchieri F. Expression of 60-kD heat shock protein increases during carcinogenesis in the uterine exocervix. Pathobiology. 2002;70:83–88. doi: 10.1159/000067304. [DOI] [PubMed] [Google Scholar]
- Cappello F, Rappa F, David S, Anzalone R, Zummo G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003;23:1325–1331. [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, David S, Anzalone R, Zummo G. Ten kilodalton heat shock protein (HSP10) is overexpressed during carcinogenesis of large bowel and uterine exocervix. Cancer Lett. 2003;196:35–41. doi: 10.1016/S0304-3835(03)00212-X. [DOI] [PubMed] [Google Scholar]
- Cappello F, Bellafiore M, Palma A, David S, Marcianò V, Bartolotta T, Sciumè C, Modica G, Farina F, Zummo G, Bucchieri F. 60KDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur J Histochem. 2003;47:105–110. doi: 10.4081/814. [DOI] [PubMed] [Google Scholar]
- Cappello F, David S, Rappa F, Bucchieri F, Marasà L, Bartolotta TE, Farina F, Zummo G. The expression of HSP60 and HSP10 in large bowel carcinomas with lymph node metastase. BMC Cancer. 2005;5:139. doi: 10.1186/1471-2407-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Marasà L, Zummo G, Macario AJ. Hsp60 expression, new locations, functions, and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7:801–809. doi: 10.4161/cbt.7.6.6281. [DOI] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Felice V, Zummo G, Macario AJ. Chlamydia trachomatis infection and anti-Hsp60 immunity: the two sides of the coin. PLoS Pathog. 2009;5:e1000552. doi: 10.1371/journal.ppat.1000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrao S, Campanella C, Anzalone R, Farina F, Zummo G, Conway de Macario E, Macario AJ, Cappello F, Rocca G. Human Hsp10 and early pregnancy factor (EPF) and their relationship and involvement in cancer and immunity: current knowledge and perspectives. Life Sci. 2010;86:145–152. doi: 10.1016/j.lfs.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Czarnecka AM, Campanella C, Zummo G, Cappello F. Heat shock protein 10 and signal transduction: a “capsula eburnea” of carcinogenesis? Cell Stress Chaperones. 2006;11:287–294. doi: 10.1379/CSC-200.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin SM, Panaccione R. Evolving inflammatory bowel disease treatment paradigms: top-down versus step-up. Med Clin N Am. 2010;94:1–18. doi: 10.1016/j.mcna.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Endo T, Yamano K. Multiple pathways for mitochondrial protein traffic. Biol Chem. 2009;390:723–730. doi: 10.1515/BC.2009.087. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Masuda T, Ohtani H, Sasaki I, Funayama Y, Matsuno S, Nagura H. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor gamma/delta in inflammatory bowel disease. Gastroenterology. 1991;101:670–678. doi: 10.1016/0016-5085(91)90524-o. [DOI] [PubMed] [Google Scholar]
- Hansen JJ, Bross P, Westergaard M, Nielsen MN, Eiberg H, Børglum AD, Mogensen J, Kristiansen K, Bolund L, Gregersen N. Genomic structure of the human mitochondrial chaperonin genes: HSP60 and HSP10 are localised head to head on chromosome 2 separated by a bidirectional promoter. Hum Genet. 2003;112:71–77. doi: 10.1007/s00439-002-0837-9. [DOI] [PubMed] [Google Scholar]
- Henderson B, Calderwood SK, Coates AR, Cohen I, Eden W, Lehner T, Pockley AG. Caught with their PAMPs down? The extracellular signalling actions of molecular chaperones are not due to microbial contaminants. Cell Stress Chaperones. 2010;15:123–141. doi: 10.1007/s12192-009-0137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–16. doi: 10.1007/s00535-005-1744-3. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Monden T, Kanoh T, Tsujie M, Izawa H, Haba A, Ohnishi T, Sekimoto M, Tomita N, Shiozaki H, Monden M. Extraction and analysis of diagnostically useful proteins from formalin-fixed, paraffin-embedded tissue sections. J Histochem Cytochem. 1998;46:397–403. doi: 10.1177/002215549804600314. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Kurokawa MS, Yoshikawa H, Nara K, Takada E, Masuda C, Tsukikawa S, Ozaki S, Matsuda T, Suzuki N. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet's disease. Clin Exp Immunol. 2005;139:371–378. doi: 10.1111/j.1365-2249.2005.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Le TT, Dobbin CA, Banovic T, Howard CB, Flores Fde M, Vanags D, Naylor DJ, Hill GR, Suhrbier A. Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J Biol Chem. 2005;280:4037–4047. doi: 10.1074/jbc.M411569200. [DOI] [PubMed] [Google Scholar]
- Kamphuis S, Kuis W, Jager W, Teklenburg G, Massa M, Gordon G, Boerhof M, Rijkers GT, Uiterwaal CS, Otten HG, Sette A, Albani S, Prakken BJ. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet. 2005;2–8(366):50–56. doi: 10.1016/S0140-6736(05)66827-4. [DOI] [PubMed] [Google Scholar]
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, Domschke W. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- Ludwig D, Stahl M, Ibrahim ET, Wenzel BE, Drabicki D, Wecke A, Fellermann K, Stange EF. Enhanced intestinal expression of heat shock protein 70 in patients with inflammatory bowel diseases. Dig Dis Sci. 1999;44:1440–1447. doi: 10.1023/A:1026616221950. [DOI] [PubMed] [Google Scholar]
- Macario AJL, Conway de Macario E. Sick chaperones, cellular stress and disease. N Engl J Med. 2005;353:1489–1501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- Macario AJL, Conway de Macario E. Chaperonopathies by defect, excess or mistake. Ann NY Acad Sci. 2007;1113:178–191. doi: 10.1196/annals.1391.009. [DOI] [PubMed] [Google Scholar]
- Macario AJL, Cappello F, Zummo G, Conway de Macario E (2010) Chaperonopathies of senescence and the scrambling of interactions between the chaperoning and the immune systems. Ann NY Acad Sci, in press [DOI] [PubMed]
- Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circ. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- Merendino AM, Bucchieri F, Campanella C, Marcianò V, Ribbene A, David S, Zummo G, Burgio G, Corona DF, Conway de Macario E, Macario AJL, Cappello F. Hsp60 is actively secreted by human tumor cells. PLoS ONE. 2010;5:e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara K, Kurokawa MS, Chiba S, Yoshikawa H, Tsukikawa S, Matsuda T, Suzuki N. Involvement of innate immunity in the pathogenesis of intestinal Behçet's disease. Clin Exp Immunol. 2008;152:245–251. doi: 10.1111/j.1365-2249.2008.03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Osterloh A, Kalinke U, Weiss S, Fleischer B, Breloer M. Synergistic and differential modulation of immune responses by Hsp60 and lipopolysaccharide. J Biol Chem. 2007;282:4669–4680. doi: 10.1074/jbc.M608666200. [DOI] [PubMed] [Google Scholar]
- Osterloh A, Veit A, Gessner A, Fleischer B, Breloer M. Hsp60-mediated T cell stimulation is independent of TLR4 and IL-12. Int Immunol. 2008;20:433–443. doi: 10.1093/intimm/dxn003. [DOI] [PubMed] [Google Scholar]
- Otaka M, Odashima M, Watanabe S. Role of heat shock proteins (molecular chaperones) in intestinal mucosal protection. Biochem Biophys Res Commun. 2006;348:1–5. doi: 10.1016/j.bbrc.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Otani S, Otaka M, Jin M, Okuyama A, Itoh S, Iwabuchi A, Sasahara H, Itoh H, Tashima Y, Masamune O. Effect of preinduction of heat shock proteins on acetic acid-induced colitis in rats. Dig Dis Sci. 1997;42:833–846. doi: 10.1023/A:1018832618275. [DOI] [PubMed] [Google Scholar]
- Peetermans WE, D’Haens GR, Ceuppens JL, Rutgeerts P, Geboes K. Mucosal expression by B7- positive cells of the 60-Kilodalton heat-shock protein in inflammatory bowel disease. Gastroenterology. 1995;108:75–82. doi: 10.1016/0016-5085(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Petrof EO, Ciancio MJ, Chang EB. Role and regulation of intestinal epithelial heat shock proteins in health and disease. Chin J Dig Dis. 2004;5:45–50. doi: 10.1111/j.1443-9573.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Multhoff G. Cell stress proteins in extracellular fluids: friend or foe? Novartis Found Symp. 2007;291:86–100. doi: 10.1002/9780470754030.ch7. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2007;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Puga Yung GL, Fidler M, Albani E, Spermon N, Teklenburg G, Newbury R, Schechter N, Broek T, Prakken B, Billetta R, Dohil R, Albani S. Heat shock protein-derived T-cell epitopes contribute to autoimmune inflammation in pediatric Crohn’s disease. PLoS One. 2009;2(4):e7714. doi: 10.1371/journal.pone.0007714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharamurthy R, Hennigar RA, Fuchs S, Palaniswami P, Sherman M, Selvaraj P. Extravasations and emigration of neutrophils to the inflammatory site depend on the interaction of immune-complex with Fcgamma receptors and can be effectively blocked by decoy Fcgamma receptors. Blood. 2008;111:894–904. doi: 10.1182/blood-2007-04-085944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Ludwig D, Fellermann K, Stange EF. Intestinal expression of human heat shock protein 90 in patients with Crohn's disease and ulcerative colitis. Dig Dis Sci. 1998;43:1079–1087. doi: 10.1023/A:1018847205420. [DOI] [PubMed] [Google Scholar]
- Stevens TR, Winrow VR, Blake DR, Rampton DS. Circulating antibodies to heat-shock protein 60 in Crohn's disease and ulcerative colitis. Clin Exp Immunol. 1992;90:271–274. doi: 10.1111/j.1365-2249.1992.tb07941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa Y, Kamiya S, Yagita A, Sugamata M, Atomi Y. Induction of autoimmune colitis by Yersinia enterocolitica 60-kilodalton heat-shock protein. Scand J Gastroenterol. 2000;35:1188–1193. doi: 10.1080/003655200750056673. [DOI] [PubMed] [Google Scholar]
- Tatsuta T. Protein quality control in mitochondria. J Biochem. 2009;146:455–461. doi: 10.1093/jb/mvp122. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden W. Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol Rev. 1991;121:5–28. doi: 10.1111/j.1600-065X.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Eden W, Wick G, Albani S, Stress CI. Heat shock proteins, and autoimmunity how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- Viera AJ, Garrett JM. Understanding interobserver agreement: the Kappa Statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–2429. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- Wang Y, Whittall T, McGowan E, Younson J, Kelly C, Bergmeier LA, Singh M, Lehner T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;174:3306–3316. doi: 10.4049/jimmunol.174.6.3306. [DOI] [PubMed] [Google Scholar]