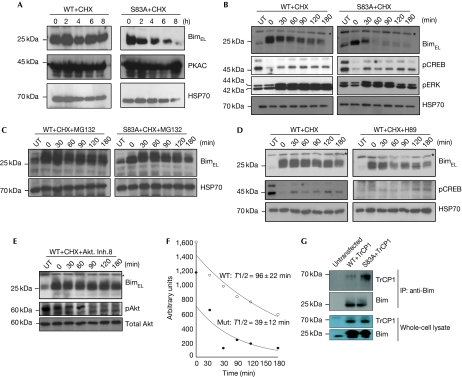
Figure 3.
Protein kinase A-mediated phosphorylation controls the stability of BimEL protein. (A) HEK 293T cells were transfected with constructs encoding either the wild type or BimEL (S83A) and after 48 h were treated with 10 μM CHX to inhibit protein synthesis. Samples were collected at the indicated time points and subjected to western blot analysis for the indicated proteins. (B) MEFs expressing either the WT or the mutant protein were treated with CHX and samples were collected at the indicated time points and subjected to western blot analysis for the indicated proteins. (C) The experiment was carried out as in B, but with the addition of the proteasomal inhibitor MG132 (40 μM). (D,E) MEFs expressing wild-type mouse BimEL were treated with CHX ± the PKA inhibitor H89 (20 μM), or with the Akt inhibitor VIII (5 μM, Calbiochem) and lysates were analysed by western blotting for the indicated proteins. Asterisks indicate a nonspecific band. (F) Half-life measurement of the wild-type and PKA phosphorylation site mutant S83A mouse BimEL proteins in MEFs, using the same experimental setting as in B. (G) HEK 293T cells were co-transfected with constructs encoding TrCP1 and with either the WT or the S83A mutant form of BimEL. Lysates were immunoprecipitated with Bim-specific antibodies and probed for βTrCP (upper panel). Total protein expression is shown in the lower panel. cAMP, cyclic AMP; CHX, cycloheximide; CREB, cAMP response element-binding; Fsk, forskolin; MEF, mouse embryonic fibroblast; RNAi, RNA interference; UT, cells not treated with 4-OHT; WT, wild-type.