Abstract
OBJECTIVE
The optimal treatment of hyperglycemia in general surgical patients with type 2 diabetes mellitus is not known.
RESEARCH DESIGN AND METHODS
This randomized multicenter trial compared the safety and efficacy of a basal-bolus insulin regimen with glargine once daily and glulisine before meals (n = 104) to sliding scale regular insulin (SSI) four times daily (n = 107) in patients with type 2 diabetes mellitus undergoing general surgery. Outcomes included differences in daily blood glucose (BG) and a composite of postoperative complications including wound infection, pneumonia, bacteremia, and respiratory and acute renal failure.
RESULTS
The mean daily glucose concentration after the 1st day of basal-bolus insulin and SSI was 145 ± 32 mg/dL and 172 ± 47 mg/dL, respectively (P < 0.01). Glucose readings <140 mg/dL were recorded in 55% of patients in basal-bolus and 31% in the SSI group (P < 0.001). There were reductions with basal-bolus as compared with SSI in the composite outcome [24.3 and 8.6%; odds ratio 3.39 (95% CI 1.50–7.65); P = 0.003]. Glucose <70 mg/dL was reported in 23.1% of patients in the basal-bolus group and 4.7% in the SSI group (P < 0.001), but there were no significant differences in the frequency of BG <40 mg/dL between groups (P = 0.057).
CONCLUSIONS
Basal-bolus treatment with glargine once daily plus glulisine before meals improved glycemic control and reduced hospital complications compared with SSI in general surgery patients. Our study indicates that a basal-bolus insulin regimen is preferred over SSI in the hospital management of general surgery patients with type 2 diabetes.
Patients with diabetes are more likely to undergo surgery than people without diabetes (1). Surgery in diabetic patients is associated with longer hospital stay, greater perioperative morbidity and mortality, and higher health care resource utilization than nondiabetic subjects (1,2). Increased morbidity and mortality in diabetic patients relates in part to higher incidence of comorbid conditions including coronary heart disease, hypertension, and renal insufficiency (1,3,4), as well as adverse effects of hyperglycemia on clinical outcome (5,6). The strongest evidence that hyperglycemia worsens outcomes is from cardiac surgery and critically ill patients admitted to surgical intensive care units (ICU) (7–9). In this setting, observational and prospective clinical trials have shown that hyperglycemia is associated with increased rates of hospital complications and mortality (8,10,11) and that improved glycemic control reduces multiorgan failure, systemic infections, and short- and long-term mortality (7,8).
Several observational studies in general surgery patients admitted to noncritical care areas have also shown that hyperglycemia is associated with increased risks of perioperative complications, length of stay, and mortality (12–14). Despite the increased risk of perioperative complications, hyperglycemia is frequently overlooked and inadequately addressed because of fear of hypoglycemia (9,15). In the presence of altered nutrition during the perioperative period, outpatient antidiabetic regimens are frequently held while initiating sliding scale regular insulin (SSI) coverage, a practice associated with limited therapeutic success (16,17). Reports from academic institutions have shown that most patients are treated with SSI and that basal insulin is prescribed in less than half of patients (18). We recently reported that in general medicine patients with type 2 diabetes mellitus, treatment with basal-bolus insulin regimen improved glycemic control without increasing the risk of severe hypoglycemia compared with NPH and regular insulin twice daily (19) and with SSI regimen (20). However, no previous prospective randomized trials evaluated the optimal management of hyperglycemia in patients undergoing general surgery. Accordingly, this study compared the efficacy and safety of a basal-bolus insulin regimen and SSI in general surgery patients with type 2 diabetes mellitus.
RESEARCH DESIGN AND METHODS
Adult patients admitted to undergo general elective or emergency surgery and not expected to require ICU admission were eligible for inclusion. We enrolled patients with a blood glucose (BG) level between 140 mg/dL and 400 mg/dL who had a history of diabetes for more than 3 months, aged 18–80 years old, treated with diet alone, any combination of oral antidiabetic agents, or low-dose insulin therapy at a daily dose ≤0.4 units/kg before admission. Exclusion criteria included hyperglycemia without a known history of diabetes, cardiac surgery, clinically relevant hepatic disease or impaired renal function (serum creatinine ≥ 3.0 mg/dL), history of diabetic ketoacidosis (21), pregnancy, and any mental condition rendering the subject unable to give informed consent.
This study was conducted at Grady Memorial Hospital, a community teaching hospital; Emory University Hospital, a tertiary referral academic institution; and the Veterans Administration Medical Center, a government healthcare teaching hospital in Atlanta, Georgia. The study protocol and consent were approved by the institutional review boards at Emory University. Treatment assignment was coordinated by a research pharmacist at each institution following a computer-generated block randomization table. All patients were managed for medical and surgical problem(s) by their primary care team who received a copy of the assigned treatment protocol. Management of the insulin regimen was directed by the study team. A teaching endocrinologist rounded daily with the research team and was available for diabetes care consultation. Patients were contacted by telephone or returned for an outpatient visit within 1 month after discharge to determine the rate of infection and postoperative complications.
The goal of insulin therapy was to maintain fasting and premeal glucose concentration between 100 and 140 mg/dL. Patients were randomly assigned to receive either a basal-bolus regimen with insulins glargine and glulisine (Lantus and Apidra, Sanofi-Aventis) or to SSI with regular (Novolin R, Novo Nordisk) insulin. Oral antidiabetic drugs were discontinued on admission. Patients treated with basal-bolus therapy were started at a total daily dose (TDD) of 0.5 units/kg divided half as insulin glargine once daily and the other half as insulin glulisine given before meals. If a patient was not able to eat, insulin glargine was given but insulin glulisine was held until meals were resumed. The insulin TDD was reduced to 0.3 units/kg in patients ≥70 years of age and/or with a serum creatinine ≥2.0 mg/dL. Patients randomized to SSI received regular insulin four times daily for BG >140 mg/dL. The doses of insulin were adjusted according to a prespecified protocol (Supplementary Table 1). For subjects receiving SSI, if the mean daily BG level was >240 mg/dL, or if three consecutive values were >240 mg/dL on the maximal sliding scale dose, patients were switched to basal-bolus regimen starting at a TDD of 0.5 units/kg.
Outcome measures
The primary outcomes of the study were differences between treatment groups in mean daily BG concentration and a composite of postoperative complications including wound infection, pneumonia, bacteremia, respiratory failure, and acute renal failure. Secondary outcomes included differences between treatment groups in any of the following measures: occurrence of mild and severe hypoglycemia (<70 mg/dL and <40 mg/dL, respectively), length of hospital stay, surgical complications (wound infection and dehiscence, bacteremia, pneumonia, and acute renal failure defined as an increased in serum creatinine >50% of baseline and/or a serum creatinine >2.5 mg/dL), admission to the ICU, and death.
Statistical analysis
The baseline and outcome variables were compared with the use of Wilcoxon tests and χ2 tests (or Fisher exact test) as appropriate. Power calculation was conducted based on our previous RABBIT 2 medicine study (19), which showed a mean daily BG difference of >30 mg/dL between basal-bolus with insulin analog versus SSI regimens. Assuming a within-group standard deviation of 40 mg/dL and α-error rate of 5% and a <10% attrition rate, we estimated that 104 subjects per group were needed to achieve 90% power. Statistical analysis was performed using the SAS (version 9.2; SAS Institute, Cary, NC). A P value of <0.05 was considered significant. The data are presented as means ± SD.
RESULTS
From February 2008 to October 2009, 234 patients consented to participate. Of them, 23 patients were excluded after randomization because they received <24 h of insulin treatment (n = 14), treatment with continuous insulin infusion during parenteral nutrition (n = 3), consent withdrawal (n = 3), or cancelled surgery (n = 3). A total of 104 patients in the basal-bolus regimen and 107 patients in the SSI group were included in analysis (Supplementary Fig. 1). Of them, 105 patients were recruited at Grady Memorial Hospital, 101 patients at Emory University Hospital, and five patients at the Veterans Administration Medical Center. The groups were well matched, because the characteristics of the patients did not differ on sex, age, racial distribution, BMI, or duration of diabetes (Table 1). On admission, 17.1% of patients were treated with diet alone, 63% with oral agents alone, 9.5% with combination of oral agents and insulin, and 10.4% with insulin alone.
Table 1.
Clinical characteristics on admission, type of surgery, and blood glucose values during treatment
| All | SSI | Basal-bolus insulin | P value | |
|---|---|---|---|---|
| Number of patients | 211 | 107 | 104 | NS |
| Male/female | 107/104 | 53/54 | 54/50 | NS |
| Race (white/black/other) | 75/117/19 | 40/59/8 | 35/58/11 | NS |
| Age (years) | 58 ± 11 | 57 ± 10 | 58 ± 12 | NS |
| BMI (kg/m2) | 31.3 ± 8.0 | 32.2 ± 8.5 | 30.3 ± 7.4 | NS |
| Body weight (kg) | 90.5 ± 24.1 | 93.1 ± 25.6 | 87.9 ± 22.2 | NS |
| Duration diabetes mellitus (years) | 6.5 ± 6.3 | 6.8 ± 6.3 | 6.3 ± 6.2 | NS |
| Serum creatinine (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.3 | NS |
| Diabetic treatment on admission | ||||
| Diet alone | 17 | 11 | 6 | NS |
| Oral agents | 153 | 80 | 73 | NS |
| Insulin alone | 22 | 11 | 11 | NS |
| Insulin + oral agents | 20 | 11 | 9 | NS |
| Type of surgery | ||||
| Cancer | 76 | 40 | 36 | NS |
| Gastrointestinal/genitourinary benign | 59 | 28 | 31 | NS |
| Vascular | 31 | 15 | 16 | NS |
| Trauma | 38 | 20 | 18 | NS |
| Others | 7 | 5 | 2 | NS |
| BG values | ||||
| Admission (mg/dL) | 190 ± 92 | 184 ± 80 | 197 ± 104 | NS |
| Randomization | 198 ± 54 | 194 ± 56 | 202 ± 51 | NS |
| Presurgery (mg/dL) | 178 ± 71 | 181 ± 72 | 174 ± 70 | NS |
| Postsurgery (mg/dL) | 198 ± 53 | 195 ± 52 | 201 ± 55 | NS |
| After 2nd day of Rx | 159 ± 42 | 172 ± 46 | 145 ± 32 | <0.001 |
| BG values after 24-h treatment, % readings | ||||
| <140 mg/dL | 41.9 ± 30.9 | 31.2 ± 28 | 52.9 ± 30.1 | <0.001 |
| 70–140 mg/dL | 41.6 ± 30.3 | 31.7 ± 28.1 | 51.8 ± 29.2 | <0.001 |
| >180 mg/dL | 28.1 ± 30.7 | 35.3 ± 33.5 | 20.5 ± 25.5 | <0.001 |
The mean admission glucose for the entire cohort was 190 ± 92 mg/dL and the mean A1C was 7.72 ± 2.2%. The mean admission BG and A1C concentration in the basal-bolus group (197 ± 104 mg/dL, A1C 8.08 ± 2.4%) were higher than in SSI group (184 ± 80 mg/dL and 7.38 ± 1.9%, respectively), but differences did not reach statistical significance (P = 0.548 and P = 0.070). The mean BG at randomization in the basal-bolus group was 202 ± 51 mg/dL and in the SSI group was 194 ± 56 mg/dL, and the mean glucose before surgery was 178 ± 71 mg/dL and increased to 198 ± 53 after surgery (P < 0.001) with similar rise in glucose in both groups.
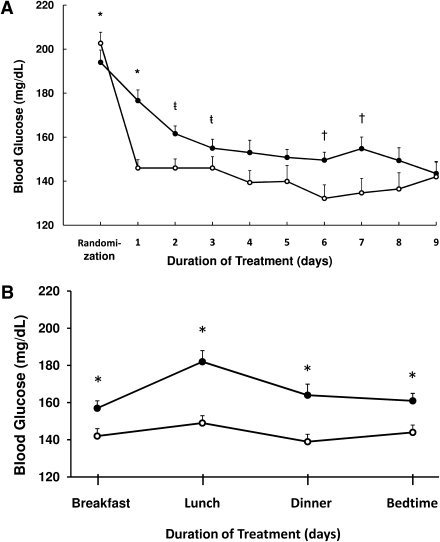
Patients treated with insulin glargine and glulisine had better glycemic control than SSI (P < 0.01) (Fig. 1A). When compared with SSI regimen, treatment with basal-bolus insulin resulted in significantly lower mean fasting glucose (155 ± 37 mg/dL vs. 165 ± 40 mg/dL; P = 0.037) and mean daily glucose during the hospital stay (157 ± 32 mg/dL vs. 176 ± 44 mg/dL; P < 0.001). The mean BG level after the 1st day of therapy was 145 ± 32 mg/dL in glargine/glulisine group and 172 ± 47 mg/dL in SSI group (P < 0.01). The percentages of glucose readings <140 mg/dL were higher in basal-bolus than in SSI treatment group (53 ± 30 vs. 31 ± 28%; P < 0.001).
Figure 1.
A: Glucose levels during basal-bolus and SSI treatment. Changes in blood glucose concentration after the 1st day of treatment with basal-bolus with glargine once daily plus glulisine before meals (○) and with SSI 4-times daily (●). *P < 0.001, ŧP = 0.02, †P = 0.01. B: Glucose levels before meals and bedtime. Premeal and bedtime glucose levels were higher throughout the day in the SSI group (●) compared with basal-bolus regimen (○).
Premeal glucose levels before meals and at bedtime were significantly higher in the SSI group compared with basal-bolus regimen (Fig. 1B). In addition, 13 patients (12%) treated with SSI remained with persistent hyperglycemia (BG >240 mg/dL) despite increasing the SSI dose to the maximal or insulin-resistant scale (Supplementary Fig. 2). Glycemic control in the SSI failure subjects rapidly improved after they were switched to basal-bolus regimen. SSI failure subjects had a higher mean admission glucose (242 ± 95 mg/dL vs. 175 ± 74 mg/dL; P = 0.127) and developed wound infection at a higher rate (30.8 vs. 7.5%; P = 0.027).
Difference between groups in the frequency of the composite outcome including wound infection, pneumonia, bacteremia, respiratory failure, and acute renal failure were higher in the SSI group (24.3%) than in basal-bolus group (8.6%; P = 0.003) (Table 2). There were reductions with basal-bolus as compared with SSI in wound infection (2.9 vs. 10.3%; P = 0.05), pneumonia (0 vs. 2.8%; P = 0.247), and acute renal failure (3.8 vs. 10.3%; P = 0.106). In addition, the basal-bolus regimen resulted in lower but not significant reduction in postsurgical ICU admissions. A total of 13 out of 104 patients treated with basal-bolus insulin (12.5%) and 21 out of 107 patients treated with SSI (19.6%) required admission to the ICU (P = 0.16). The length of ICU stay was shorter in patients treated with basal-bolus insulin compared with SSI group (3.19 ± 2.14 vs. 1.23 ± 0.60; P = 0.003). There were no differences in hospital length of stay (9.4 ± 12.8 vs. 9.1 ± 6.8 days; P = 0.25) or in mortality (one patient in each arm) between groups.
Table 2.
Composite hospital complications and outcomes composite hospital complications
| All | SSI | Basal-bolus insulin | P value | |
|---|---|---|---|---|
| Wound infections | 14 | 11 | 3 | 0.050 |
| Pneumonia | 3 | 3 | 0 | 0.247 |
| Acute respiratory failure | 6 | 5 | 1 | 0.213 |
| Acute renal failure | 15 | 11 | 4 | 0.106 |
| Bacteremia | 3 | 2 | 1 | 0.999 |
| Number of patients with complications | 35 | 26 | 9 | 0.003 |
| Mortality | 2 | 1 | 1 | NS |
| Postsurgery ICU admission (%) | 16 | 19.6 | 12.5 | NS |
| Length of stay (days) | ||||
| ICU | 2.51 ± 1.90 | 3.19 ± 2.14 | 1.23 ± 0.60 | 0.003 |
| Hospital | 6.8 ± 8.9 | 6.3 ± 5.6 | 7.23 ± 11.39 | NS |
The average total insulin use after 24-h treatment was 33.4 units/day in the basal-bolus group and 12.3 units/day in the SSI group (P < 0.001). For the basal-bolus group, after 24-h treatment, the mean dose of insulin glargine was 21.8 ± 8.6 units/day and of glulisine was 14.8 ± 7.6 units/day, and the mean supplemental (correction) dose was 8.7 ± 4.4 units/day. Patients treated with SSI received a mean daily dose of 12.3 ± 6.5 units of regular insulin (range 9.7 to 14.4 units) after 24-h treatment, with 88.5% of patients receiving less than 20 units and 39.4% receiving less than 10 units per day.
Hypoglycemia (<70 mg/dL) occurred in 23.1% of patients in the basal-bolus and 4.7% of patients in the SSI treated group (P < 0.001) (Table 3). Severe hypoglycemia (<40 mg/dL) was reported in 3.8% of patients in the basal-bolus and none in the SSI group (P = 0.057). There were no differences in the frequency of hypoglycemia between patients treated with insulin before admission compared with insulin-naïve patients. A glucose <70 and <40 mg/dL was observed in 11.9 and 4.8% of those treated with insulin before admission compared with 11.8 and 1.2% of insulin-naïve patients.
Table 3.
Hypoglycemic events
| Variable | All | SSI | Basal-bolus insulin | P value |
|---|---|---|---|---|
| Number of patients | 211 | 107 | 104 | |
| Number of BG tests | 3,778 | 1,826 | 1,952 | |
| BG <40 mg/dL | ||||
| Number of patients (%) | 4 (3.8) | 0 (0) | 4 (3.8) | 0.057 |
| Number of events | 4 | 0 | 4 | |
| Number of readings (%) | 0.10 | 0 | 0.20 | |
| BG <60 mg/dL | ||||
| Number of patients (%) | 14 (6.6) | 2 (1.9) | 12 (11.5) | 0.005 |
| Number of events | 17 | 2 | 15 | |
| Number of readings (%) | 0.45 | 0.11 | 0.77 | |
| BG <70 mg/dL | ||||
| Number of patients (%) | 29 (0.8) | 5 (4.7) | 24 (23) | <0.001 |
| Number of events | 44 | 6 | 38 | |
| Number of readings (%) | 1.16 | 0.33 | 1.95 |
A total of 15 patients >70 years of age or with a serum creatinine >2 mg/dL were treated with an initial TDD of 0.3 unit/kg. When compared with patients treated with an initial TDD of 0.3 unit/kg, patients receiving 0.5 unit/kg had no differences in mean daily BG (159 ± 33 mg/dL and 147 ± 25 mg/dL, respectively; P = 0.19) or in the frequency of hypoglycemic events (23.6 and 20.0%; P > 0.99). In addition, 18 patients (8.5%) were started on insulin before surgery (mean ± SD 3.8 ± 5 days; median 1.5 days) and 193 patients (91.5%) were started the day of or after surgery (mean ± SD 1.6 ± 1 days; median 1 day). When compared with patients treated with insulin before surgery, those who received insulin after surgery had no significant differences in the mean daily BG (166.7 ± 40.5 mg/dL vs. 163.9 ± 29.6 mg/dL; P = 0.93) or in the frequency of hypoglycemic events (14.0 vs. 11.1%; P > 0.99).
CONCLUSIONS
This prospective, randomized clinical trial compared the glycemic efficacy and safety of a basal-bolus regimen with insulin glargine once daily and insulin glulisine before meals to SSI in general surgery patients with type 2 diabetes mellitus. We observed that basal-bolus treatment significantly improved glycemic control measured as mean daily glucose concentration after the 1st day of therapy and reduced perioperative complications observed as the composite of postoperative complications including wound infection, pneumonia, bacteremia, respiratory failure, and acute renal failure. We conclude that basal-bolus insulin regimen is preferred over SSI in the hospital management of general surgery patients.
The association between hyperglycemia and increased risk of hospital complications and mortality is well established in ICU and cardiac surgery patients (7–9). In non-ICU patients, small observational studies have also shown that perioperative hyperglycemia is associated with increased risk of infectious complications and mortality (13,14). General surgery patients with glucose levels of >12.2 mmol/L (>220 mg/dL) on the first postoperative day had a 2.7 times increased rate of infection (13). Another study reported that patients with glucose levels of 5.6–11.1 mmol/L (110–200 mg/dL) and those with glucose levels of >11.1 mmol/L had, respectively, 1.7-fold and 2.1-fold increased mortality compared with those with glucose levels <5.6 mmol/L (13). Most patients with diabetes admitted to general surgery service have poor glycemic control, and diabetes management is frequently overlooked (6,22). In the presence of altered nutrition, physicians hold their patient’s outpatient antidiabetic regimen and initiate sliding scale insulin coverage (16,17,23). The University Health System Consortium Benchmarking Project (24), an alliance of 90 academic health centers across the U.S., showed that in the non-ICU setting, subcutaneous insulin therapy was prescribed only in 45% of patients, with a range of 12–77% across measured hospitals.
We recently reported the results of the RABBIT 2 medicine trial, a prospective multicenter trial comparing the efficacy and safety of a basal-bolus insulin regimen with glargine and glulisine insulin and SSI in insulin-naïve patients with type 2 diabetes mellitus admitted to general medicine wards (20). We achieved a glucose target of <140 mg/dL in 55% of patients in the basal-bolus and 31% in the SSI group (P < 0.001). The results of the RABBIT surgery trial also indicate that a basal-bolus insulin regimen is more effective than SSI in general surgery patients. In addition, we observed a significant reduction between groups in the frequency of the composite outcome including wound infection, pneumonia, bacteremia, respiratory failure, and acute renal failure. Taken together, these two studies indicate that a basal-bolus insulin regimen is preferable over SSI in medical and surgical patients with type 2 diabetes mellitus and clearly indicate that SSI alone should not be used in the management of hospitalized subjects with diabetes.
The basal-bolus regimen with glargine once daily and glulisine before meals at a starting dose of 0.3–0.5 unit/kg/day is well tolerated with an acceptable rate of hypoglycemia. In the RABBIT medicine trial, only two patients (3%) in the glargine and glulisine group experienced a BG <60 mg/dL and no patients had a value <40 mg/dL. In this RABBIT surgery trial, a glucose <70 mg/dL was reported in 23.1% of patients (1.9% of glucose readings) in the basal-bolus and in 4.7% (0.3% of readings) in the SSI group (P = <0.001), but there were no significant differences in the frequency of severe hypoglycemia. Differences in hypoglycemic events between the two trials could be in part explained by reduced nutritional intake in surgical patients and the fact that in the previous trial we dosed the TDD of insulin as 0.4 units/kg for BG between 140 mg/dL and 200 mg/dL and 0.5 unit/kg for BG between 200 mg/dL and <400 mg/dL. In the RABBIT surgery trial most patients received a single daily dose of 0.5 units/kg.
We acknowledge the following limitations in this study. We excluded patients undergoing cardiac surgery or in need for ICU care and with clinically relevant hepatic disease or with serum creatinine ≥3.0 mg/dL and history of hyperglycemic crises. In addition, we limited the recruitment to patients treated with diet, oral antidiabetic agents, and a low-dose insulin therapy and excluded patients receiving a TDD >0.4 unit/kg per day before admission. In such patients, higher insulin doses may be needed to achieve glycemic control. A large prospective, multicenter, randomized clinical trial of glycemic control in general surgery setting is certainly needed to address these important issues. Such studies should include additional treatment regimes comparing basal insulin alone (glargine, detemir, or NPH) and basal-bolus regimens in surgical patients with type 2 diabetes.
In summary, basal-bolus insulin with glargine once daily plus glulisine before meals represents a simple and an effective regimen for the management of general surgery patients with type 2 diabetes mellitus. The basal-bolus regimen is associated with better glycemic control and lower frequency of hospital complications than SSI, without increasing the number of severe hypoglycemic events. These results indicate that a basal-bolus insulin regimen should be preferred over SSI treatment in general surgery patients and that SSI alone should not be used in the management of hospitalized subjects with diabetes.
Acknowledgments
This investigator-initiated study was supported by an unrestricted grant from sanofi-aventis (Bridgewater, NJ). G.E.U. is supported by American Diabetes Association Research Grant 7-03-CR-35, National Institutes of Health (NIH) Grant R03-DK-073190-01, and General Clinical Research Center Grant M01 RR-00039. D.S. is supported by NIH Research Grant K12-RR-017643.
No other potential conflicts of interest relevant to this article were reported.
The sponsors of the study were not involved in the study design, data collection, analysis, or interpretation of the results or preparation of the article.
G.E.U., D.S., S.J., L.P., A.T., P.M., D.U., C.N., D.O., and M.R. researched the data. G.E.U., D.S., S.J., L.P., C.N., D.O., and M.R. also reviewed and edited the manuscript and contributed to the discussion. G.E.U. wrote the primary portion of the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1407/-/DC1.
Clinical trial reg. no. NCT00596687, clinicaltrials.gov.
References
- 1.Clement S, Braithwaite SS, Magee MF, et al. American Diabetes Association Diabetes in Hospitals Writing Committee Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004;27:553–591 [DOI] [PubMed] [Google Scholar]
- 2.Smiley DD, Umpierrez GE. Perioperative glucose control in the diabetic or nondiabetic patient. South Med J 2006;99:580–591 [DOI] [PubMed] [Google Scholar]
- 3.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004;109:1497–1502 [DOI] [PubMed] [Google Scholar]
- 4.Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 2008;248:585–591 [DOI] [PubMed] [Google Scholar]
- 5.Risum O, Abdelnoor M, Svennevig JL, et al. Diabetes mellitus and morbidity and mortality risks after coronary artery bypass surgery. Scand J Thorac Cardiovasc Surg 1996;30:71–75 [DOI] [PubMed] [Google Scholar]
- 6.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125:1007–1021 [DOI] [PubMed] [Google Scholar]
- 8.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 9.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 2006;355:1903–1911 [DOI] [PubMed] [Google Scholar]
- 10.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 11.Kitabchi AE, Freire AX, Umpierrez GE. Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients. Metabolism 2008;57:116–120 [DOI] [PubMed] [Google Scholar]
- 12.Turnbull PJ, Sinclair AJ. Evaluation of nutritional status and its relationship with functional status in older citizens with diabetes mellitus using the mini nutritional assessment (MNA) tool—a preliminary investigation. J Nutr Health Aging 2002;6:185–189 [PubMed] [Google Scholar]
- 13.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 1998;22:77–81 [DOI] [PubMed] [Google Scholar]
- 14.Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol 2007;156:137–142 [DOI] [PubMed] [Google Scholar]
- 15.Malouf R, Brust JC. Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol 1985;17:421–430 [DOI] [PubMed] [Google Scholar]
- 16.Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J 1994;14:313–322 [PubMed] [Google Scholar]
- 17.Hirsch IB. Sliding scale insulin—time to stop sliding. JAMA 2009;301:213–214 [DOI] [PubMed] [Google Scholar]
- 18.Schnipper JL, Barsky EE, Shaykevich S, Fitzmaurice G, Pendergrass ML. Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital. J Hosp Med 2006;1:145–150 [DOI] [PubMed] [Google Scholar]
- 19.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 21.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:2739–2748 [DOI] [PubMed] [Google Scholar]
- 22.Levetan CS, Magee MF. Hospital management of diabetes. Endocrinol Metab Clin North Am 2000;29:745–770 [DOI] [PubMed] [Google Scholar]
- 23.Umpierrez G, Maynard G. Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity? J Hosp Med 2006;1:141–144 [DOI] [PubMed] [Google Scholar]
- 24.Boord JB, Greevy RA, Braithwaite SS, et al. Evaluation of hospital glycemic control at US academic medical centers. J Hosp Med 2009;4:35–44 [DOI] [PubMed] [Google Scholar]