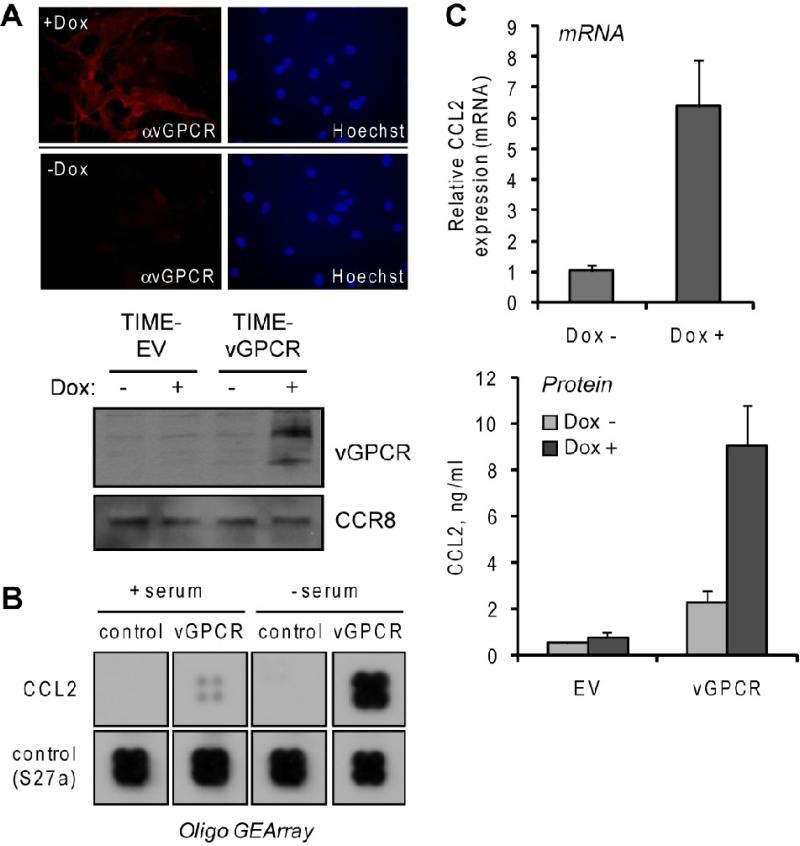
Figure 1.
Induction of CCL2 in vGPCR-transduced endothelial cells. (A) Testing of Tet-responsive element (TRE)-vGPCR transduced, rtTA transactivator-expressing TIME cells for Dox-inducible expression of vGPCR, visualized by using vGPCR rabbit antiserum (Chiou et al., 2002) in indirect immunofluorescence assay (top) and immunoblotting (bottom). EV, empty vector (B) Membrane hybridization analysis of CCL2 mRNA expression in TIME-vGPCR cells treated with Dox (vGPCR+) or mock treated (control) for 24 h. Cells were either maintained in medium containing serum or serum-starved (to remove possible effects of serum-contained factors) for 24 h prior to addition of Dox. Extracted total RNA from these cultures were used to generate biotin-labeled cRNA probes for array hybridization (Materials and Methods). Sections of the array corresponding to CCL2 and “housekeeping” normalization control (RPSS27a, ribosomal protein) are shown. (C) Verification of array data for CCL2 induction by SYBR green-based qRT-PCR analysis of CCL2 mRNA levels (top) and ELISA quantitation of secreted protein (bottom) in Dox treated and untreated TIME-vGPCR cultures. qRT-PCR values were normalized to 18S RNA levels determined in parallel real-time PCR reactions; bars indicate deviations from the mean values of paired PCR reactions. For ELISA, duplicate cultures were analyzed and protein concentrations (in culture media) calculated from parallel standard-curve values.