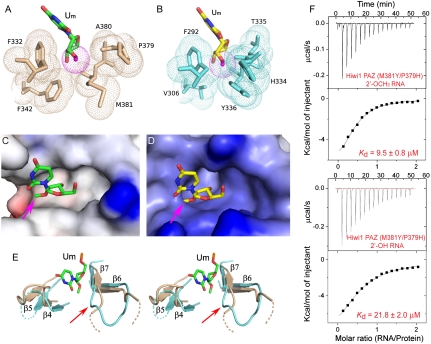
Fig. 4.
Methyl group of 2′-OCH3 can be accommodated within the Hiwi1 but not Ago1 PAZ-binding pockets. (A and B) Binding pocket residues surrounding the methyl group of 2′-OCH3 at the RNA 3′ end in Hiwi1 PAZ (A) and Ago1 PAZ (B, modeled 2′-OCH3 to replace 2′-OH observed in the crystal structure) RNA complexes. Binding pocket residues in Hiwi1 PAZ (in biscuit, A) and Ago1 PAZ (in cyan, B) complexes are highlighted as dotted balls in a van der Waals radius representation. The 2′-OCH3 residues (dotted ball) are shown in green for the Hiwi1 PAZ-RNA complex (A) and in yellow for the Ago1 PAZ-RNA complex (B). (C and D) Electrostatic surface representation of Hiwi1 PAZ (C) and Ago1 PAZ (D) binding pockets in their RNA complexes containing 2′-OCH3 and 2′OH (replaced by 2′-OCH3) at their 3′ ends respectively. The Hiwi1 PAZ-binding pocket can readily accommodate the methyl group (shown by pink arrow) of 2′-OCH3 of Um at the 3′ end, but the more constricted Ago1 PAZ-binding pocket is unable, due to steric clashes, to accommodate the methyl group. (E) A stereo view of the relative arrangements of β6-β7 strands and β4-β5 strands of Hiwi1 PAZ (in biscuit) and Ago1 PAZ (in blue) in their RNA-bound complexes. The 2′-OCH3-modified Um14 residue in the Hiwi1 PAZ-RNA complex is shown in green. (F) ITC measurements for binding of Hiwi1 (M381Y/P379H) PAZ to self-complementary 14-mer RNA (12-bp + 2-nt overhang) containing 2′-OCH3 (Top) and 2′-OH (Bottom) at the 3′ end.