Abstract
Despite the importance of the nitrogen (N) cycle on marine productivity, little is known about variability in N sources and cycling in the ocean in relation to natural and anthropogenic climate change. Beyond the last few decades of scientific observation, knowledge depends largely on proxy records derived from nitrogen stable isotopes (δ15N) preserved in sediments and other bioarchives. Traditional bulk δ15N measurements, however, represent the combined influence of N source and subsequent trophic transfers, often confounding environmental interpretation. Recently, compound-specific analysis of individual amino acids (δ15N-AA) has been shown as a means to deconvolve trophic level versus N source effects on the δ15N variability of bulk organic matter. Here, we demonstrate the first use of δ15N-AA in a paleoceanographic study, through analysis of annually secreted growth rings preserved in the organic endoskeletons of deep-sea gorgonian corals. In the Northwest Atlantic off Nova Scotia, coral δ15N is correlated with increasing presence of subtropical versus subpolar slope waters over the twentieth century. By using the new δ15N-AA approach to control for variable trophic processing, we are able to interpret coral bulk δ15N values as a proxy for nitrate source and, hence, slope water source partitioning. We conclude that the persistence of the warm, nutrient-rich regime since the early 1970s is largely unique in the context of the last approximately 1,800 yr. This evidence suggests that nutrient variability in this region is coordinated with recent changes in global climate and underscores the broad potential of δ15N-AA for paleoceanographic studies of the marine N cycle.
Keywords: compound-specific isotope analysis, deep-sea corals, nitrogen cycle, North Atlantic Oscillation, stable N isotopes
As evidence for anthropogenic climate change continues to mount (1), there is growing need for understanding its effects on the productivity of marine ecosystems. This understanding is critically dependent on long-term datasets derived from natural archives. Proxy data from sediment cores satisfies the need for long records, but detailed temporal resolution can be compromised by low sedimentation rates and bioturbation. Alternatively, long-lived, annually layered, biogenic archives such as corals and bivalves may be used to determine long-term (centuries to millennia) variability at a resolution comparable to that of modern observations. Biogenic archives have been used extensively for isotopic and trace element-based (e.g., δ18O, Mg/Ca) reconstructions of physical oceanographic variables such as temperature and salinity (2). In comparison, reconstructions of ecologically important variables such as nutrient source, primary productivity, and flux and composition of export production remain more difficult, due to lack of well preserved organic samples and geochemical complexity of organic material.
The δ15N of organic material has long been used as a tracer of both trophic level of organisms and of the source of nitrogen (N) at the base of the food web (3). However, inability to distinguish between these influences with measurements of bulk δ15N can be a major barrier to interpretation. Recently, the common protein amino acids (AA) have been shown to fall into two groups with divergent isotopic enrichment behavior during trophic transfer. One group of “trophic” amino acids (Tr-AA) becomes strongly enriched in 15N with each trophic transfer, while a second group of “source” amino acids (Src-AA) have δ15N values that undergo little or no change (4, 5). The use of compound-specific δ15N analysis of AA (δ15N-AA) to deconvolute the effects of trophic transfer on bulk δ15N signals has been demonstrated in a number of environments (5–8). Here, for the first time, we apply δ15N-AA measurements to a paleo-archive (coral) in order to resolve a major issue in the interpretation of bulk δ15N records from a marine ecosystem. Specifically, we distinguish between temporal (decadal, centennial) change in fixed nitrogen source, versus change in export production trophic structure in the Scotia-Maine region of the Northwest Atlantic Ocean.
Our analysis focuses on specimens of the deep-sea gorgonian coral Primnoa resedaeformis with a two-part endoskeleton of calcite and proteinaceous “gorgonin.” The diagenetically stable gorgonin (9) is deposited in discrete annual growth rings, permitting annually resolved sampling over the decadal to centennial life spans of individual colonies (10, 11). Whereas the calcite is synthesized from dissolved inorganic carbon at depth, the gorgonin derives from particulate organic matter (POM), which the corals feed on, having been recently biosynthesized and exported from surface waters. In this regard, gorgonian corals act like “living sediment traps,” which can be used for δ15N-based reconstructions of changes in euphotic zone nutrients and export production back through time in open and/or remote regions of the ocean (12–14).
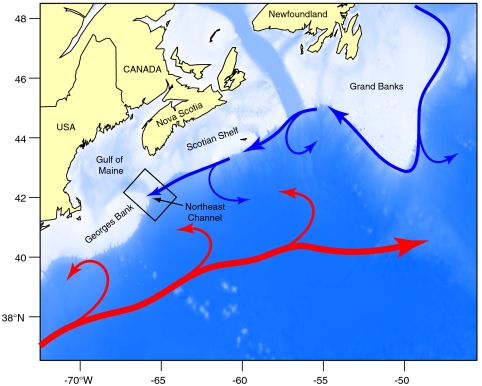
Specimens were collected from the outer Northeast Channel, on the upper continental slope southwest of Nova Scotia, Canada (Fig. 1). Here, climate forcing associated with the North Atlantic Oscillation (NAO) (15) drives the ebb and flow of subpolar and subtropical slopewater masses (16–19). During negative modes of the NAO, cold (6 °C), fresh (34.9 psu), and nutrient-poor (∼15 μM 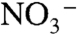 ) Labrador Slope Water (LSW) is advected along slope, from the Grand Banks of Newfoundland to southwestward of Georges Bank (Fig. 1). During positive modes of the NAO, LSW retreats northeastward and is replaced by relatively warm (10 °C), saline (35.2 psu) and nutrient-rich (∼23 μM
) Labrador Slope Water (LSW) is advected along slope, from the Grand Banks of Newfoundland to southwestward of Georges Bank (Fig. 1). During positive modes of the NAO, LSW retreats northeastward and is replaced by relatively warm (10 °C), saline (35.2 psu) and nutrient-rich (∼23 μM 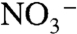 ) Warm Slope Water (WSW) associated with the Gulf Stream. Modal shifts in slopewater regime may impact the δ15N of export production through (i) changes in the δ15N of nitrate (
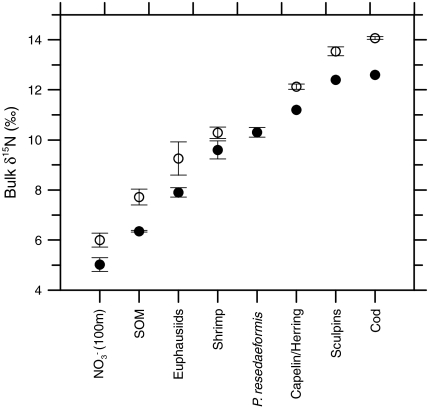
) Warm Slope Water (WSW) associated with the Gulf Stream. Modal shifts in slopewater regime may impact the δ15N of export production through (i) changes in the δ15N of nitrate (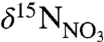 ), which averages 1‰ higher in LSW than in WSW (Fig. 2), and (ii) physical oceanographic-driven changes in phytoplankton and zooplankton community structure (20–22). To resolve this fundamental ambiguity in distinguishing nutrient sources from trophic effects, we present detailed bulk δ15N records supplemented with measurements of δ15N-AA.
), which averages 1‰ higher in LSW than in WSW (Fig. 2), and (ii) physical oceanographic-driven changes in phytoplankton and zooplankton community structure (20–22). To resolve this fundamental ambiguity in distinguishing nutrient sources from trophic effects, we present detailed bulk δ15N records supplemented with measurements of δ15N-AA.
Fig. 1.
Map of the study area with location of Northeast Channel. Blue arrows mark the approximate position of the Labrador Current, which transports Labrador slope water (LSW) southward, along-slope. Red arrows show the location of the Gulf Stream. Warm slope water (WSW) occupies the region between the Gulf Stream and continental shelf edge. Temperature and nitrate data were extracted from the 2 × 2 degree grid centered over the Northeast Channel, from 150 to 250 m water depth, where interannual variability is most pronounced (18). Temperature data are from the Bedford Institute of Oceanography hydrographic database. Nutrient data are from the Biochem database of Fisheries and Oceans Canada (44).
Fig. 2.
Differences in the δ15N of 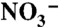 (
(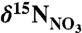 ), sedimentary organic matter (SOM; reflects the long-term average for sinking particles), invertebrates (whole body), and fish (muscle), from (•) Scotian Shelf/Gulf of Maine (WSW influenced) and (○) Newfoundland and Labrador (LSW influenced) regions. The
), sedimentary organic matter (SOM; reflects the long-term average for sinking particles), invertebrates (whole body), and fish (muscle), from (•) Scotian Shelf/Gulf of Maine (WSW influenced) and (○) Newfoundland and Labrador (LSW influenced) regions. The 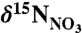 data are from this study. Data for Gulf of Maine SOM (45), Georges Bank fish and invertebrates (29), tissue samples of Northeast Channel P. resedaeformis (13), outer Newfoundland and Labrador shelf and slope SOM (46), fish and invertebrates (Bonavista corridor and Hawke Channel) (30) were compiled from the literature. Specimens of P. resedaeformis from comparable LSW-influence off Newfoundland have not been measured. Error bars are standard errors of the mean (where n > 2).
data are from this study. Data for Gulf of Maine SOM (45), Georges Bank fish and invertebrates (29), tissue samples of Northeast Channel P. resedaeformis (13), outer Newfoundland and Labrador shelf and slope SOM (46), fish and invertebrates (Bonavista corridor and Hawke Channel) (30) were compiled from the literature. Specimens of P. resedaeformis from comparable LSW-influence off Newfoundland have not been measured. Error bars are standard errors of the mean (where n > 2).
Results
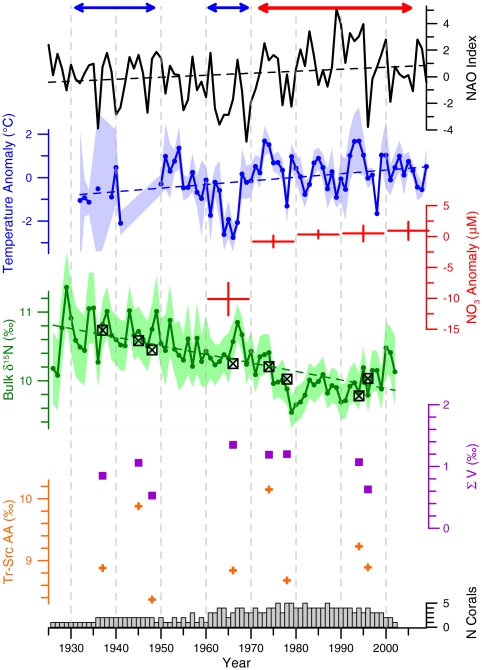
The alternating presence of LSW and WSW at the Northeast Channel is evident in the subsurface (150–250 m depth) temperature record, which is positively correlated with the NAO index at lags of 1–2 yr (Fig. 3) (16, 19). Prior to 1970, both NAO and temperature persisted in a mostly negative/cold regime culminating with the 1960s, the coldest decade of the instrumental record. Since the early 1970s, NAO and temperature have shifted to a mostly positive/warm regime. Although too sparse for meaningful time-series statistics, subsurface nitrate concentration data show a similar pattern of low values in the 1960s, followed by higher values from 1970 onward. While NAO index, temperature, and nitrate increased over the twentieth century, the bulk δ15N record from P. resedaeformis significantly decreased by about 1‰ over the same period, with most of the decline occurring after 1970 (Table 1). Moreover, interannual variability in bulk δ15N was significantly and inversely correlated with NAO and temperature at mutually consistent temporal lags (Fig. 3 and SI Text).
Fig. 3.
Time series of the winter (December to March) station-based NAO index (15); temperature and nitrate (plotted as the annual means of monthly anomalies to reduce the effects of seasonal cycles); and annual mean bulk δ15N from six colonies of the deep-sea gorgonian P. resedaeformis. Shaded areas represent 95% confidence intervals around annual means. Dashed lines indicate long-term trends, where significant. Note the cold periods (blue bars) of the 1930s/1940s and 1960s and sustained warm period (red bar) since 1970. Bulk δ15N is most strongly correlated with NAO at a lag of 4 years (r = -0.19, p < 0.10) and with temperature at a lag of 3 years (r = -0.27, p < 0.05). Cross-correlation analysis technique is described in SI Text. Also shown are ΣV and a trophic index based on the broadest possible grouping of Tr-AAs and Src-AAs (see text for details). Squares in bulk δ15N plot show values of the eight individual samples used for δ15N-AA analysis.
Table 1.
Linear regression statistics for time-series records of NAO, temperature, bulk δ15N, and compound-specific isotope analysis (CSIA) parameters
| Parameter | Length of record (years AD) | N | Slope | St. Error | r2 | p-value |
| NAO index | 1926–2001 | 76 | 0.020 | 0.010 | 0.05 | 0.056 |
| Temperature index | 1931–2001 | 59 | 0.019 | 0.007 | 0.12 | 0.008 |
| Bulk δ15N (all samples pooled) | 1926–2001 | 76 | −0.013 | 0.001 | 0.57 | < 0.001 |
| Bulk δ15N (samples for CSIA) | 1937–1996 | 8 | −0.014 | 0.002 | 0.91 | < 0.001 |
| TL (Glu-Phe) | 1937–1996 | 8 | 0.014 | 0.022 | 0.06 | 0.551 |
| TL (TrAA*-SrcAA†) | 1937–1996 | 8 | 0.001 | 0.011 | 0.00 | 0.927 |
| ΣV ‡ | 1937–1996 | 8 | 0.001 | 0.005 | 0.02 | 0.734 |
To maintain consistency with the coral data, NAO, and temperature time series were clipped to the time period 1926–2001. δ15N statistics include the pooled record from all specimens of P. resedaeformis, and the eight individual samples used for CSIA. Relative trophic level (TL) formulations include δ15N differences in Glu and Phe only (Glu-Phe) and also the broadest possible groupings of trophic (Tr) and source (Src) AAs (TrAA-SrcAA).
*TrAA = average(Asp,Glu,Ala,Ile,Leu,Val and Pro)
†SrcAA = average(Gly,Lys,Ser,Phe,Tyr)
‡ΣV = 1/nΣAbs(χAA), where χ (deviation) of each AA = [δ15N-AA - AVGδ15N(Ala,Asp,Glu,Ile,Leu, and Pro)], and n = the total number of AAs used in the calculation.
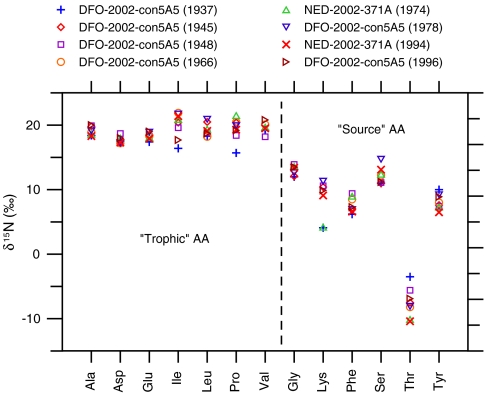
The more time-consuming analysis of δ15N-AA was performed on a subset of samples isolated from two of the longest-lived specimens. As expected from prior literature, δ15N-AA values clearly fall into two groups (Fig. 4). The Tr-AA are substantially enriched in δ15N versus the Src-AA, reflecting both autotrophic base metabolic patterns, as well as subsequent trophic transfers (4–8, 23). We note that within the Src-AA, Thr appears to constitute an outlier, with very negative values. While this is in contrast with the fundamental “source” designation, in fact similar observations for Thr (only) have been made in other animals (24), together suggesting that Thr displays a unique behavior and probably does not belong in the Src-AA grouping (25). The overall patterns in P. resedaeformis are very similar to those observed in pure plankton samples (4, 7), indicating that the basic photoautotrophic patterns are well preserved during POM transit and trophic incorporation into coral gorgonin. This qualitative observation is confirmed by the ΣV, a parameter tied to microbial resynthesis and alteration of δ15N-AA patterns (7). The ΣV values in P. resedaeformis all fall in the range expected for fresh biomass (i.e., live phytoplankton and zooplankton; 0.53 to 1.35), and further there is no significant trend in ΣV in our samples (Table 1 and Fig. 3).
Fig. 4.
Measured δ15N of individual amino acids (AAs) in eight different samples isolated from two different specimens of P. resedaeformis. Trophic-AA (those that enrich strongly with trophic transfer) and Source-AA (those that are largely stable) refer to the two amino acid categories discussed in the text; individual AA abbreviations are as defined in text.
Based on previous studies, the difference between δ15N of the Tr-AA and Src-AA provides an internally normalized indication of relative trophic position, unaffected by changes in source nutrients (4, 5). We used two different formulations to test for changes in relative trophic level. The first calculates the difference in δ15N between glutamic acid (Glu) and phenylalanine (Phe), the most common trophic level formulation based on the observation that Glu and Phe exhibit, respectively, the most and least trophic N-isotope fractionation (4). However, a formulation relying on only two AAs can be highly susceptible to diagenetic alteration or error in any single value (7). Our second formulation therefore calculates the difference in the averages of the broadest possible groupings of trophic [aspartic acid (Asp), Glu, alanine (Ala), isoleucine (Ile), leucine (Leu), valine (Val), proline (Pro)] and source [glycine (Gly), lysine (Lys), serine (Ser), Phe, tyrosine (Tyr)] AAs (7). In both formulations, regression over the record indicates no significant trend (Table 1) and the data are remarkably consistent [less than ± 1‰, or well within typical instrumental error for δ15N-AA values; see Materials and Methods and Fig. 3]. For two data points (1945 and 1975), however, values are elevated by about 1‰. These points may indicate trophic position elevation for average exported POM during these years but do not indicate any longer-term trend that might be correlated to bulk 15N values.
Discussion
Differences in nutrient processing histories underlie the distinct end-member 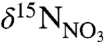 signatures between WSW and LSW. The
signatures between WSW and LSW. The 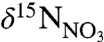 of WSW (5.02 ± 0.27‰) is identical to the nominal mean ocean value of 5‰ (26), reflecting its origin in the nutrient-rich waters beneath the Gulf Stream (27, 28). The
of WSW (5.02 ± 0.27‰) is identical to the nominal mean ocean value of 5‰ (26), reflecting its origin in the nutrient-rich waters beneath the Gulf Stream (27, 28). The 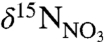 of LSW (6.00 ± 0.28‰) reflects the low nutrient surface currents from which it is formed in the northern Labrador Sea. Because LSW is depleted in nutrients and not replenished by upwelling, the residual nitrate is enriched over that of WSW by 1‰. These end-member
of LSW (6.00 ± 0.28‰) reflects the low nutrient surface currents from which it is formed in the northern Labrador Sea. Because LSW is depleted in nutrients and not replenished by upwelling, the residual nitrate is enriched over that of WSW by 1‰. These end-member 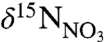 signatures reflect basin-scale circulation and likely did not vary significantly over the late Holocene.
signatures reflect basin-scale circulation and likely did not vary significantly over the late Holocene.
The 1‰ difference in 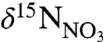 is remarkably well preserved through all levels of the food web at each of the different source water regions, from primary producers to top predators (Fig. 2). Based on an approximate 3‰ increase in bulk δ15N with each step in trophic level (29, 30), P. resedaeformis (tissue δ15N = 10.31 ± 0.19) likely feeds opportunistically on a mixture of sinking POM and small zooplankton, as previously noted (13). By this mechanism,
is remarkably well preserved through all levels of the food web at each of the different source water regions, from primary producers to top predators (Fig. 2). Based on an approximate 3‰ increase in bulk δ15N with each step in trophic level (29, 30), P. resedaeformis (tissue δ15N = 10.31 ± 0.19) likely feeds opportunistically on a mixture of sinking POM and small zooplankton, as previously noted (13). By this mechanism, 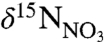 signatures in the euphotic zone are transmitted to and incorporated into the skeletal protein of deep-sea gorgonians.
signatures in the euphotic zone are transmitted to and incorporated into the skeletal protein of deep-sea gorgonians.
Processes involved in the generation of coral N-isotopic signatures on the pathway from source nutrients to the gorgonin synthesis include (i) fixed-N uptake and growth of phytoplankton, (ii) microbial reworking/resynthesis and consumption during export and sinking of POM, and (iii) the assimilation of POM and zooplankton in the coral tissue and skeleton. Disentangling the factors involved in δ15N fractionation during phytoplankton growth is simplified by the fact that nitrate utilization in the vicinity of the Northeast Channel is complete under the influence of both WSW and LSW, despite initial differences in nitrate concentrations in the two water masses (SI Text). Because the pool of nitrate is exhausted to completion, the accumulated phytoplankton biomass takes on the δ15N of the source nitrate, regardless of the species involved in nitrate assimilation or the rate of growth (31, 32). Therefore, in this system, interannual variability in coral δ15N may be attributed either to a change in δ15N of source nitrate or to changes in trophic structure of the coral’s food source.
The δ15N-AA data provide two lines of evidence that there has been no significant change in the trophic structure of the coral’s food source over the last century. First, the lack of a trend in the difference in δ15N between Tr-AA and Src-AA provides strong evidence that there was no change in trophic level over the period from 1926 to 2001, in contrast to the significant trend observed in bulk δ15N over the same time interval (Fig. 3). Second, the ΣV parameter strongly suggests that exported algal δ15N-AA patterns at this location have been preserved in these corals with little diagenetic or microbial alteration. This increases confidence in the internal measure of trophic level, and further, the lack of any trend in ΣV also suggests potential change in POM diagenetic state is not a factor. Together, these data indicate that change in 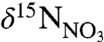 underlies the observed trends in the coral bulk δ15N record, and its inverse correlation with NAO, temperature, and nitrate. We therefore interpret the change in coral bulk δ15N over the twentieth century as a reflection of increasing presence of warm, nitrate-rich (lower
underlies the observed trends in the coral bulk δ15N record, and its inverse correlation with NAO, temperature, and nitrate. We therefore interpret the change in coral bulk δ15N over the twentieth century as a reflection of increasing presence of warm, nitrate-rich (lower 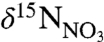 ) WSW versus that of colder, nitrate-poor (higher
) WSW versus that of colder, nitrate-poor (higher 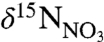 ) LSW.
) LSW.
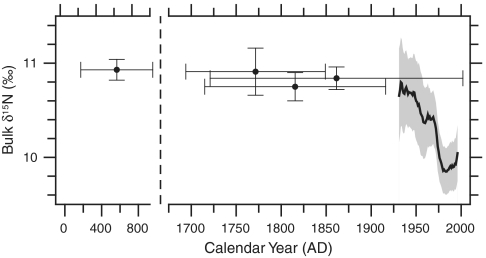
To put these data in a broader Holocene context, we also compared our twentieth century bulk δ15N record with longer-term δ15N measured over the 100- to 700-yr life spans of four fossil specimens of P. resedaeformis collected from the same location and depth in the Northeast Channel (Fig. 5). The average bulk gorgonin δ15N of these fossils, sampled at the same temporal resolution as the modern corals (approximately annual), ranged from 10.8 ± 0.06 to 11.0 ± 0.06‰ (1 SE). Although the record is not continuous, all the fossil values fall within a very similar range, and from this perspective the decline during the twentieth century record appears striking. Assuming that the extent of nitrate utilization in surface waters has not changed, this strongly suggests that the more recent decline in δ15N (implying a shift from LSW to WSW) is unique with respect to the last approximately 1,800 yr. Similarly, multiproxy evidence from sediment cores raised from the Scotian Shelf indicates near-constant temperatures for most of the past 1,600 yr, followed by sudden warming sometime within the last approximately 150 yr (33, 34). Our coral data more tightly constrain the timing of this change to the twentieth century, with most of the change occurring after 1970 when the NAO shifted to a sustained positive phase. With respect to the instrumental records, the decade of the 1960s would appear to represent an anomalous LSW-dominated period against a background of more WSW-dominated conditions. Analysis of longer-term proxy data reveals instead that LSW dominated through most of the late Holocene, and the recent shift to the WSW regime may be more similar to mid-Holocene hypsithermal conditions that ended approximately 2,500 yr ago (35).
Fig. 5.
Long-term trends in the bulk δ15N of recent and fossil (pre-AD 1900) Northeast Channel corals. Fossil corals were sampled at approximately annual resolution, and dated with conventional 14C and 210Pb dating (SI Text); horizontal error bars integrate both dating error and specimen life-span. Vertical error bars and gray shaded region represent 95% confidence intervals. Note break in x-axis scale.
This study demonstrates that δ15N-AA can be used to independently track nitrate source and trophic history in a paleoarchive, thus solving a major complication in all bulk δ15N records. The fidelity of the δ15N-AA patterns observed in this study indicates a broad range of new potential in coral-based paleoceanographic studies of the marine N cycle. Some examples of future potential include (i) the reconstruction of δ15N of average exported production from the surface ocean, independent of complicating assumptions about any single species or biomarker (36); (ii) reconstruction of phytoplankton trophic structure through time; and (iii) a possible new tracer for variations in algal community structure, based on evolving studies of the tracer potential in compound-specific stable isotope patterns (37, 38).
Our data also specifically demonstrate a unique ability of this approach in determining the relative roles of top-down (consumer-driven) versus bottom-up (resource-driven) forcings on lower trophic level ecosystems in time and space (39). The lack of any trend in trophic level or POM diagenetic state indicates that the local plankton community along the upper continental slope was unaffected or perhaps more resilient to the “trophic cascade” thought to have occurred elsewhere on the Eastern Scotian Shelf and Labrador Sea in response to overexploitation of Atlantic cod and other groundfish species (40, 41). On the other hand, whereas nitrate concentration may be impacted by internal processes, nitrate source is externally forced; our results from the Scotian/Maine upper continental slope indicate that increasing nitrate levels were associated with externally driven shifts in nitrate source partitioning. Slopewater circulation in the Scotia-Maine region is linked with broader scale climate variability associated with the North Atlantic oscillation (16, 17, 19), which has trended toward the positive modal state over the same time period that global temperatures have increased most dramatically (1). We therefore conclude that changes in nitrate source partitioning may be tied to recent, human-caused changes in global climate. These results highlight the importance of novel and creative proxies like δ15N-AA for investigating the links between climate change and ecosystem functioning beyond the last few decades of scientific observations.
Materials and Methods
Live and fossil specimens were collected by remotely operated vehicle (ROV), video grab, or scientific trawl, from approx. 42°00′N, 65°36′W, between 275 and 450 m water depth. Sections from the basal region of each colony were decalcified in 5% (v/v) HCl, and growth rings were sampled at 1 to 2 yr periodicity. Counting of growth rings and validation of their annual periodicity using bomb-14C are described elsewhere (11). Coral nitrogen isotope ratios (δ15N = ((15N/14N)sample/(15N/14N)standard - 1) × 1000) were measured using continuous flow isotope ratio mass spectrometry (CF-IRMS) and standardized relative to atmospheric N2 (AIR). Analytical error, based on sample replicates, was < 0.20‰. Data processing is described in SI Text.
Individual AA isotopic analyses on coral gorgonin were made on acid hydrolysates (6 N HCl, 100 ml, 20 h) of approximately 1 mg homogenized material, following formation of isopropyl-TFA derivatives (7, 42). Derivatives were analyzed on a Thermo Trace Ultra GC, fitted with an Agilent DB-5 column (50 m × 0.32 mm i.d. × 0.52 um film thickness), in line with an oxidation furnace and reduction furnace, and linked to a Finnigan DeltaPlus XP mass spectrometer. All samples were derivatized with an accompanying AA standard for which authentic δ15N values of each AA were determined offline, to monitor the accuracy of the instrument. Samples were injected in triplicate, bracketed by standards, and sample δ15N values were corrected based on a running average of known standard values through a run. Reproducibility for individual AA values was typically better than 1‰.
Seawater samples for 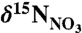 analyses were collected from three stations each off Nova Scotia (approximately 44.1°N, 58.7°W) and Labrador (54.7°N, 54.5°W) into prewashed (5% HCl and deionized water) 60 ml polyethylene bottles and frozen immediately upon collection. Measurement of
analyses were collected from three stations each off Nova Scotia (approximately 44.1°N, 58.7°W) and Labrador (54.7°N, 54.5°W) into prewashed (5% HCl and deionized water) 60 ml polyethylene bottles and frozen immediately upon collection. Measurement of 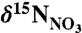 followed the bacterial denitrifier method (43), where nitrate is converted to nitrous oxide (N2O) by denitrifying bacteria that lack N2O reductase activity. Isotopic analysis (relative to AIR) was then performed on the N2O gas by CF-IRMS. Analytical error, based on sample replicates, was 0.2‰.
followed the bacterial denitrifier method (43), where nitrate is converted to nitrous oxide (N2O) by denitrifying bacteria that lack N2O reductase activity. Isotopic analysis (relative to AIR) was then performed on the N2O gas by CF-IRMS. Analytical error, based on sample replicates, was 0.2‰.
Supplementary Material
Acknowledgments.
We thank Sanford Atwood, Derek Jones, Ronnie Wolkins, Don Gordon, Pal Mortensen, Kumiko Azetsu-Scott, Kevin Pauly, and the crews of the ROPOS and CCGS Hudson, Teleost, and Martha Black for sample collection; Jennifer McKay for bulk stable isotope analyses; Tom Guilderson for radiocarbon analyses; and Evan Edinger, Richard Fairbanks, and Mike Risk for discussions. Funding was provided by the Natural Sciences and Engineering Research Council, National Science Foundation Chemical Oceanography, and the Swiss Federal Institute of Aquatic Science and Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004904108/-/DCSupplemental.
References
- 1.Allison I, et al. The Copenhagen Diagnosis, 2009: Updating the World on the Latest Climate Science. Sydney: University of New South Wales Climate Change Research Centre; 2009. [Google Scholar]
- 2.Hillaire-Marcel C, De Vernal A, editors. Proxies in Late Cenozoic Paleoceanograpy. Amsterdam: Elsevier; 2007. [Google Scholar]
- 3.Fry B. Stable Isotope Ecology. New York: Springer; 2006. [Google Scholar]
- 4.McClelland JW, Montoya JP. Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology. 2002;83:2173–2180. [Google Scholar]
- 5.McClelland JW, Holl CM, Montoya JP. Relating low delta N-15 values of zooplankton to N-2-fixation in the tropical North Atlantic: Insights provided by stable isotope ratios of amino acids. Deep-Sea Res Pt I. 2003;50:849–861. [Google Scholar]
- 6.Hannides CCS, Popp BN, Landry MR, Graham BS. Quantification of zooplankton trophic position in the North Pacific Subtropical Gyre using stable nitrogen isotopes. Limnol Oceanogr. 2009;54:50–61. [Google Scholar]
- 7.McCarthy MD, Benner R, Lee C, Fogel ML. Amino acid nitrogen isotopic fractionation patterns as indicators of heterotrophy in plankton, particulate, and dissolved organic matter. Geochim Cosmochim Acta. 2007;71:4727–4744. [Google Scholar]
- 8.Popp BN, et al. Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. Terr Ecol. 2007;1:173–190. [Google Scholar]
- 9.Sherwood OA, Scott DB, Risk MJ. Late Holocene radiocarbon and aspartic acid racemization dating of deep-sea octocorals. Geochim Cosmochim Acta. 2006;70:2806–2814. [Google Scholar]
- 10.Andrews AH, et al. Age, growth and radiometric age validation of a deep-sea, habitat-forming gorgonian (Primnoa resedaeformis) from the Gulf of Alaska. Hydrobiologia. 2002;471:101–110. [Google Scholar]
- 11.Sherwood OA, Scott DB, Risk MJ, Guilderson TP. Radiocarbon evidence for annual growth rings in the deep-sea octocoral Primnoa resedaeformis. Mar Ecol Prog Ser. 2005;301:129–134. [Google Scholar]
- 12.Heikoop JM, Hickmott DD, Risk MJ, Shearer CK, Atudorei V. Potential climate signals from the deep-sea gorgonian coral Primnoa resedaeformis. Hydrobiologia. 2002;471:117–124. [Google Scholar]
- 13.Sherwood OA, et al. Stable isotopic composition of deep-sea gorgonian corals Primnoa sp.p: A new archive of surface processes. Mar Ecol Prog Ser. 2005;301:135–148. [Google Scholar]
- 14.Williams B, Risk MJ, Ross SW, Sulak KJ. Deep-water antipatharians: Proxies of environmental change. Geology. 2006;34:773–776. [Google Scholar]
- 15.Hurrell JW, Kushnir Y, Visbeck M. Climate—the North Atlantic oscillation. Science. 2001;291:603–605. doi: 10.1126/science.1058761. [DOI] [PubMed] [Google Scholar]
- 16.Greene CH, Pershing AJ. The flip-side of the North Atlantic Oscillation and modal shifts in slope-water circulation patterns. Limnol Oceanogr. 2003;48:319–322. [Google Scholar]
- 17.Loder JW, Shore JA, Hannah CG, Petrie BD. Decadal-scale hydrographic and circulation variability in the Scotia-Maine region. Deep-Sea Res Pt II. 2001;48:3–35. [Google Scholar]
- 18.Petrie B, Drinkwater K. Temperature and salinity variability on the Scotian Shelf and in the Gulf of Maine 1945–1990. J Geophys Res. 1993;98:20079–20089. [Google Scholar]
- 19.Pershing AJ, et al. Oceanographic responses to climate in the Northwest Atlantic Ocean. Oceanography. 2001;14:76–82. [Google Scholar]
- 20.Barton AD, Greene CH, Monger BC, Pershing AJ. The Continuous Plankton Recorder survey and the North Atlantic Oscillation: Interannial- to Multidecadal-scale patterns of phytoplankton varioability in the North Atlantic Ocean. Prog Oceanogr. 2003;58:337–358. [Google Scholar]
- 21.Head EJH, Sameoto D. Inter-decadal variability in zooplankton and phytoplakton abundnace on the Newfoundland and Scotian shelves. Deep-Sea Res Pt II. 2007;54:2686–2701. [Google Scholar]
- 22.Drinkwater K, et al. The response of marine ecosystems to marine climate variability associated with the North Atlantic Oscillation. In: Hurrell JW, Kushnir Y, Ottersen G, Visbeck M, editors. The North Atlantic Oscillation: Climatic Significance and Environmental Impact. Washington: Am Geophysical Union; 2003. pp. 211–234. (Geophysical Monograph 134). [Google Scholar]
- 23.Chikaraishi Y, et al. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr-Meth. 2009;7:740–750. [Google Scholar]
- 24.Hare EP, Fogel ML, Stafford TW, Mitchell AD, Hoering TC. The isotopic composition of carbon and nitrogen in individual amino acids isolated from modern and fossil proteins. J Archaeol Sci. 1991;18:277–292. [Google Scholar]
- 25.Styring AK, Sealy JC, Evershed RP. Resolving the bulk δ15N values of ancient human and animal bone collagen via compound-specific nitrogen isotope analysis of constituent amino acids. Geochim Cosmochim Acta. (in press) [Google Scholar]
- 26.Sigman D, Altabet MA, McCorkle DC, Francois R, Fischer G. The delta N-15 of nitrate in the Southern Ocean: Nitrogen cycling and circulation in the ocean interior. J Geophys Res. 2000;105:19599–19614. [Google Scholar]
- 27.Csanady GT, Hamilton P. Circulation of slopewater. Cont Shelf Res. 1988;8:565–624. [Google Scholar]
- 28.Townsend DW, Rebuck ND, Thomas MA, Karp-Boss L, Gettings RM. A changing nutrient regime in the Gulf of Maine. Cont Shelf Res. 2010;30:820–832. [Google Scholar]
- 29.Fry B. Food web structure on Georges Bank from stable C, N, and S isotopic compositions. Limnol Oceanogr. 1988;33:1182–1190. [Google Scholar]
- 30.Sherwood GD, Rose GA. Stable isotope analysis of some representative fish and invertebrates of the Newfoundland and Labrador continental shelf food web. Estuar Coast Shelf S. 2005;63:537–549. [Google Scholar]
- 31.Altabet MA, et al. The nitrogen isotope biogeochemistry of sinking particles from the margin of the Eastern North Pacific. Deep-Sea Res Pt I. 1999;46:655–679. [Google Scholar]
- 32.Thunell R, Sigman D, Muller-Karger F, Astor Y, Varela R. Nitrogen isotope dynamics of the Cariaco Basin, Venezuela. Global Biogeochem Cy. 2004;18:GB3001. [Google Scholar]
- 33.Keigwin LD, Sachs JP, Rosenthal Y. A 1600-year history of the Labrador Current off Nova Scotia. Clim Dynam. 2003;21:53–62. [Google Scholar]
- 34.Levac E. High resolution Holocene palynological record from the Scotian Shelf. Mar Micropaleontol. 2001;43:179–197. [Google Scholar]
- 35.Scott DB, Baki V, Younger C. Late Pleistocene-Holocene paleoceanographic changes on the eastern Canadian continental margin. Palaeogeogr Palaeoclimatol Palaeoecol. 1989;74:279–295. [Google Scholar]
- 36.Higgins MB, Robinson RS, Carter SJ, Pearson A. Evidence from chlorin nitrogen isotopes for alternating nutrient regimes in the Eastern Mediterranean. Earth Planet Sc Lett. 2010;290:102–107. [Google Scholar]
- 37.Larsen T, Taylor DL, Leigh MB, O’Brien DM. Stable isotope fingerprinting: A novel method for identifying plant, fungal, or bacterial origins of amino acids. Ecology. 2009;90:3526–3535. doi: 10.1890/08-1695.1. [DOI] [PubMed] [Google Scholar]
- 38.Lehman J. Santa Cruz: University of California; 2009. Compound-specific amino acid isotopes as tracers of algal central metabolism: developing new tools for tracing prokaryotic vs. eukaryotic primary production and organic nitrogen in the ocean; p. 90. PhD thesis. [Google Scholar]
- 39.Frank KT, Petrie B, Shackell NL. The ups and downs of trophic control in continental shelf ecosystems. Trends Ecol Evol. 2007;22:236–242. doi: 10.1016/j.tree.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Frank KT, Petrie B, Choi JS, Leggett WC. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- 41.Worm B, Myers RA. Meta-analysis of cod-shrimp interactions reveals top-down control in oceanic food webs. Ecology. 2003;84:162–173. [Google Scholar]
- 42.Silfer JA, Engel MH, Macko SA, Jumeau EJ. Stable carbon isotope analysis of amino acid enantiomers by conventional isotope ratio mass spectrometry and combined gas chromatograph/isotope ratio mass spectrometry. Anal Chem. 1991;63:370–374. [Google Scholar]
- 43.Sigman DM, et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem. 2001;73:4145–4153. doi: 10.1021/ac010088e. [DOI] [PubMed] [Google Scholar]
- 44.Gregory D, Narayanan S. Biochem: A national archive for marine biology and chemistry data. AZMP Bull #3. www.meds-sdmm.dfo-mpo.gc.ca/isdm-gdsi/azmp-pmza/documents/docs/bulletin_3_04.pdf.
- 45.Macko SA. Austin: University of Texas; 1981. Stable nitrogen isotope ratios as tracers of organic geochemical processes; p. 182. PhD thesis. [Google Scholar]
- 46.Muzuka ANN, Hillaire-Marcel C. Burial rates of organic matter along the eastern Canadian margin and stable isotope constraints on its origin and diagenetic evolution. Mar Geol. 1999;160:251–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.