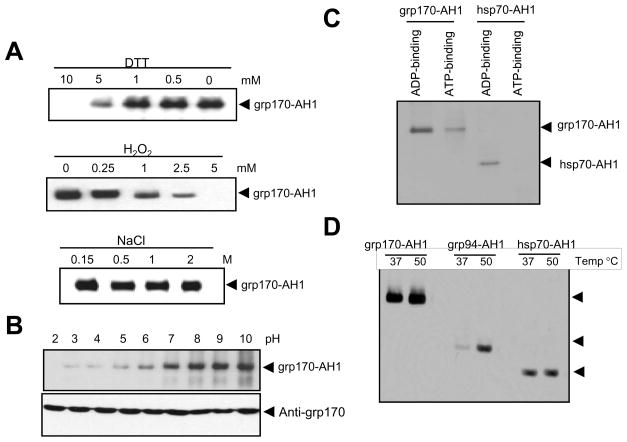
Figure 2. Large stress protein grp170 is more efficient in binding peptides than other chaperones.
A. Effect of reducing, oxidative agents and salt concentrations on the complex formation. Grp170 was incubated with biotinylated AH1 peptides in the presence of DTT, H2O2 or NaCl at different concentrations as indicated. B. pH effect on the complex formation. Grp170 was incubated with biotinylated AH1 peptides in PBS with different pH, and analyzed by immunoblot. Membranes were stripped and re-probed with antibodies against grp170. C. Effect of ATP/ADP on grp170-AH1 peptide complex. Grp170- and hsp70-AH1 peptide complexes were generated by incubation of chaperones with biotinylated AH1(18mer) peptide at 37°C. The AH1 complexes were loaded onto ADP-agarose (lane 1 and 3) or ATP-agarose (lane 2 and 4) column, followed by washing with 500 mM NaCl containing PBS. Grp170 or hsp70 was eluted from column with PBS containing 5 mM ADP (lane 1 and 3) or ATP (lane 2 and 4). Eluted fraction was examined by immunoblot using streptavidin-HRP. D. Comparison of peptide binding ability of different stress proteins. Grp170, gp96/grp94 or hsp70 was incubated with biotinylated AH1 peptide in PBS at 37°C or 50 °C for 30 min, followed by incubation at room temperature for additional 1h. Representative data from three independent experiments are shown.