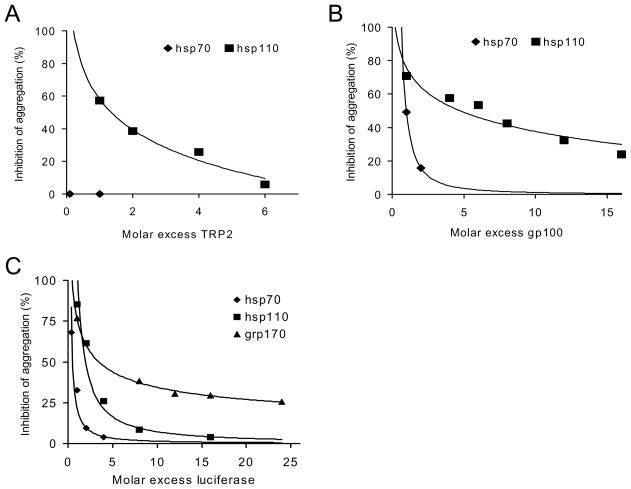
Figure 5. Large stress proteins display highly efficient antigen-holding capability.
A and B. Effective prevention of tumor protein antigen aggregation by hsp110 compared to hsp70. Increasing concentrations of (A) gp100, and (B) TRP2 was incubated with 150 nM of hsp70, or hsp110 under heat shock conditions. Protein denaturation was monitored by measuring optical density at 320nm. The optical density of the substrate heated alone at 30 min was set to 100%. Each point represents inhibition of aggregation at 30 min time point with increasing molar ratio of substrate:chaperone. C. A greater holding capacity of grp170 compared to other chaperones. Increasing concentrations of luciferase was added to each chaperone, heated at 43°C and assessed for aggregation as described. Data shown are representative of two independent experiments.