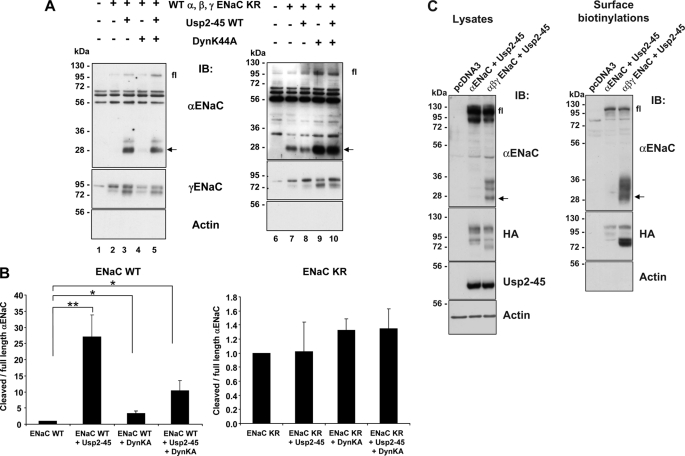
FIGURE 3.
Effect of Usp2-45 and DynK44A on proteolytic cleavage of either wild-type or KR channel. A, Hek293 cells were transiently transfected with either wild-type or cytoplasmic lysine mutant ENaC together with Usp2-45, with dominant negative dynamin (DynK44A) or both. 24 h after transfection, cells were biotinylated and biotinylated proteins analyzed as in Fig. 2 with either anti α-, γENaC, or anti-actin antibodies. Fl: full-length αENaC; arrow: cleaved αENaC. B, full-length αENaC and its cleaved fragment were quantified and the ratio of cleaved to full-length is displayed (n = 3 experiments; *, p < 0.05; **, p < 0.01 versus wt ENaC). C, Hek293 cells were transiently transfected with αENaC WT + Usp2-45 or αβγENaC WT + Usp2-45. 24 h after transfection, cells were biotinylated then lysed. Surface proteins were recovered, run on SDS/PAGE, and analyzed by Western blot as indicated (n = 3).