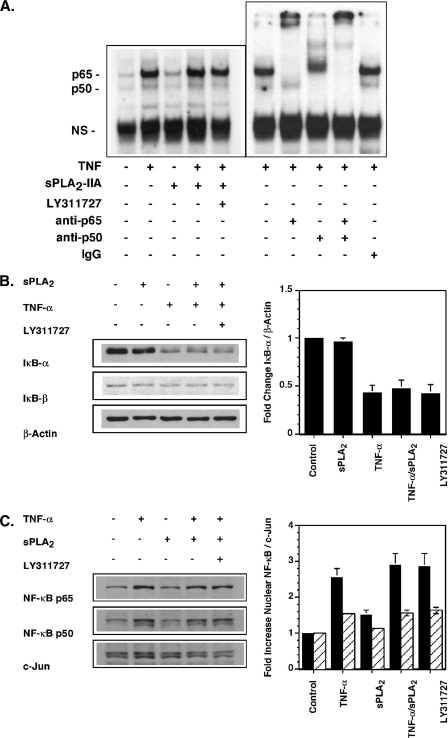
FIGURE 5.
sPLA2-IIA does not activate or enhance TNF activation of the NF-κB pathway in RSF. A, EMSA. Single flasks of 90% confluent FLSs were stimulated for 1 h in DMEM/Ham's F-12 containing 0.1% BSA with TNF (10 pg/ml), sPLA2-IIA (5 μg/ml), TNF/sPLA2-IIA or TNF/sPLA2-IIA with LY311727 (10 μm). Nuclear protein extracts were prepared and binding to a radiolabeled NF-κB consensus binding sequence was determined by EMSA as described under “Experimental Procedures.” Binding specificity to detected bands was confirmed by supershift assays with anti-p65 or anti-p50 Abs relative to an isotype-matched control Ab in extracts of TNF-stimulated cells as indicated. B, IκBα degradation. Single flasks of 90% confluent FLSs (n = 3) in DMEM/Ham's F-12 containing 0.1% BSA were stimulated for 15 min with sPLA2-IIA (5 μg/ml), TNF (50 pg/ml), TNF/sPLA2-IIA or TNF/sPLA2-IIA with LY311727 (10 μm). Total cell lysates were prepared. IκBα, IκBβ, and β-actin protein were detected by Western blot analysis. The ratio of IκBα to β-actin protein was quantified by densitometry and is normalized to the ratio measured in unstimulated cells. Data are mean ± S.E. (n = 3). C, nuclear NF-κB p65 and p50 protein in FLSs. Single flasks of 90% confluent RSF (n = 3) in DMEM/Ham's F-12 containing 0.1% BSA were stimulated for 1 h with TNF (10 pg/ml), sPLA2-IIA (5 μg/ml), TNF/sPLA2-IIA or TNF/sPLA2-IIA with LY311727 (10 μm). Nuclear protein extracts were prepared. Nuclear c-Jun, NF-κB p65 and p50 protein were detected by Western blot analysis. The ratio of nuclear NF-κB p65 and p50 to c-Jun protein was quantified by densitometry and normalized to the ratio measured in unstimulated cells. Data are mean ± S.E. (n = 3 independent FLS cultures).