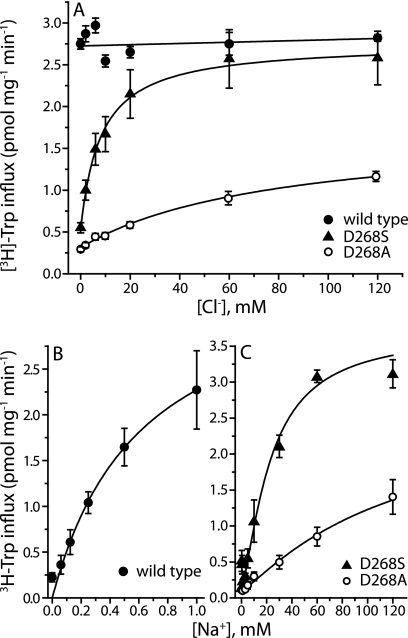
FIGURE 3.
Substitution of Asp-268 with a serine renders TnaT chloride-dependent. A, initial transport rates of [3H]tryptophan were obtained for wild type TnaT (filled circles) and mutants D268S (triangles) and D268A (open circles) over a range of Cl− concentrations (0–120 mm, with gluconate replacing Cl−). Mutation of Asp-268 to serine introduced a Cl− requirement for transport, and mutation to alanine led to a profound increase in the Km for Cl− (Table 2). B and C, mutation of Asp-268 also affected the Km for Na+ which increased from 0.6 ± 0.11 mm in wild type (B in the absence of Cl−) to 22.1 ± 2.4 mm in D268S and 286 ± 51 mm in D268A (C in the presence of saturating Cl−). Na+ was substituted with NMDG (B and C) and Cl− with SO42− (B) or gluconate (C). SO42− and gluconate were equally good as inert Cl− replacements. Each value represents the mean and S.E. of three independent experiments, each of which was performed in triplicate or quadruplicate wells.