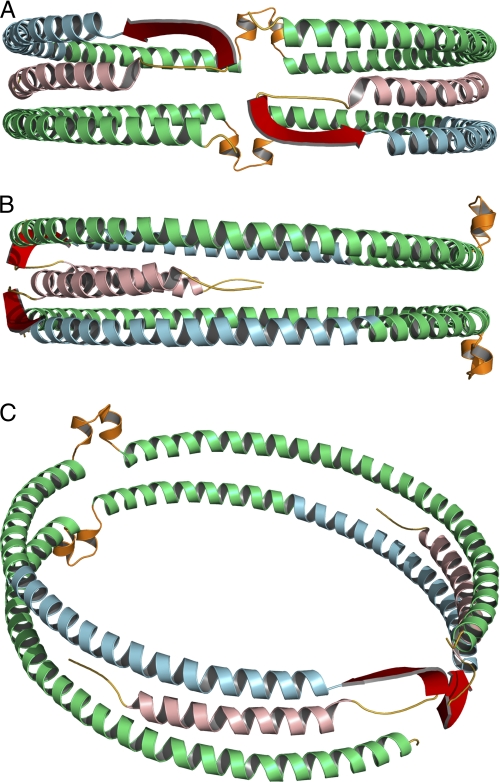
FIGURE 4.
Full-length model of apoA-I on 9.6 nm rHDL. Green, residues derived from Segrest et al. (38) model (99–243). Secondary structure domains derived from the current EPR analysis are color-coded as follows: yellow, unstructured residues 1–5 and 35–39; light red, helical residues 6–34; blue, helical residues 50–98; red, β-strand residues 40–49. The loop (orange) of the looped-belt model is at position 133–146 (14). A, front view, front of the protein complex is arbitrarily designated as the N-terminal region of the protein. B, side view, side of the protein complex is arbitrarily designated as a position parallel to the lipid bilayer of the rHDL disc and 90° relative to the N terminus. C, oblique view.