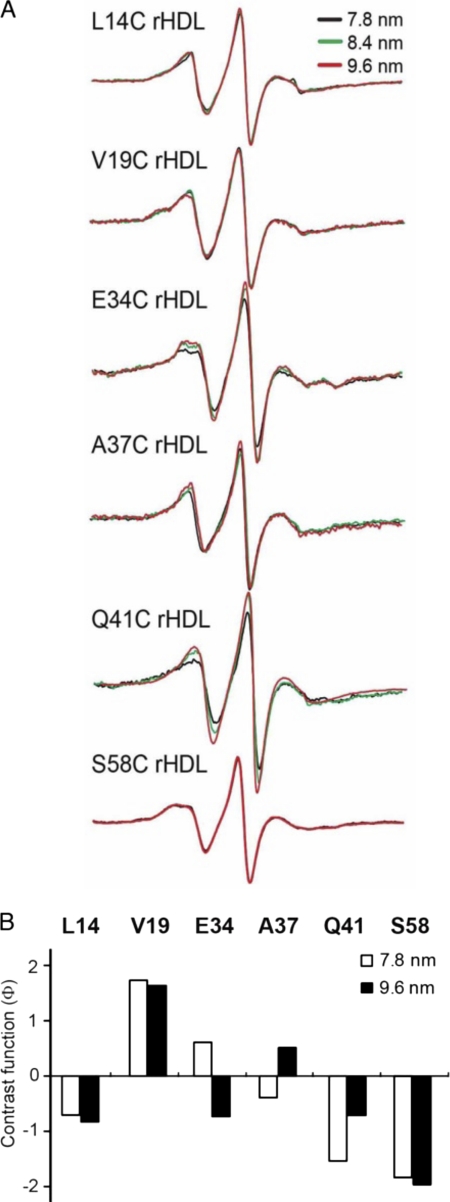
FIGURE 6.
Adaptation of the N-terminal domain of apoA-I to different rHLD sizes. Single spin-labeled apoA-I in rHDL particles of defined sizes (7.8, 8.4, and 9.6 nm diameter) were analyzed by EPR spectroscopy. Spin-labeled positions were chosen to represent regions of random coil (residues 34 and 37) and β-strand (residue 41) structure, as well as two highly immobilized residues (14 and 19) in the hydrophobic/hydrophilic interface of helix 6-34 and a highly immobilized residue (58) in helix 50-98. A, EPR spectra of spin-labeled proteins on different rHDL sizes. B, bars represent the contrast value (Φ) (Table 1) for the different rHDL sizes. A low, negative value indicates a polar environment, and a positive value represents a hydrophobic milieu.