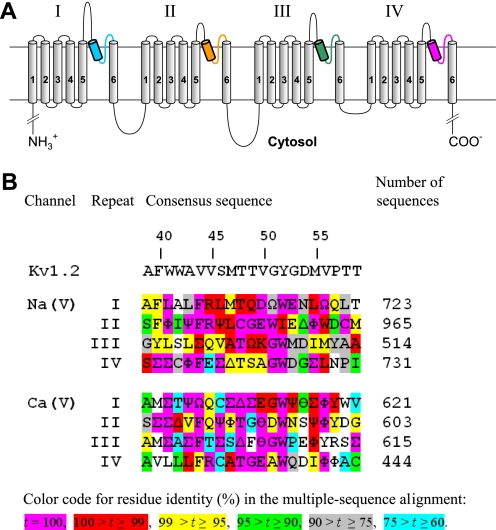
FIGURE 1.
A, transmembrane topology of the pore-forming α1-subunit in calcium and sodium channels. The α1 subunit has four repeats. Each repeat contains six transmembrane helices (S1-S6), and a P-loop between S5 and S6 helices. The homology models in this study are composed of highlighted P-helices and ascending limbs, which contribute to the P-loops. P-helices and ascending limbs are colored as in the NaV1.4 models (Figs. 2–5). B, residue conservation in P-loops of sodium and calcium channels. Each position shows the dominant residue in the multiple sequence alignments obtained with query sequences CAC1C_Human (CaV1.2) and SCN4A_HUMAN (NaV1.4) for sodium and calcium channels, respectively. Greek letters encode residues of similar physico-chemical properties: ϴ = E/D (acidic), Ω = Y/F (aromatic), Ψ = V/I (β-branched, hydrophobic), Φ = M/L (long hydrophobic), Σ = V/I/L/M/F (hydrophobic), Δ = S/T (hydroxy-substituted Cβ). The rightmost column shows number of aligned sequences from the translated nucleotide database (nucleotide collection) found by tblasn with the respective query sequence. Relative numbers of residues in P-loops (3) are shown above the Kv1.2 sequence.