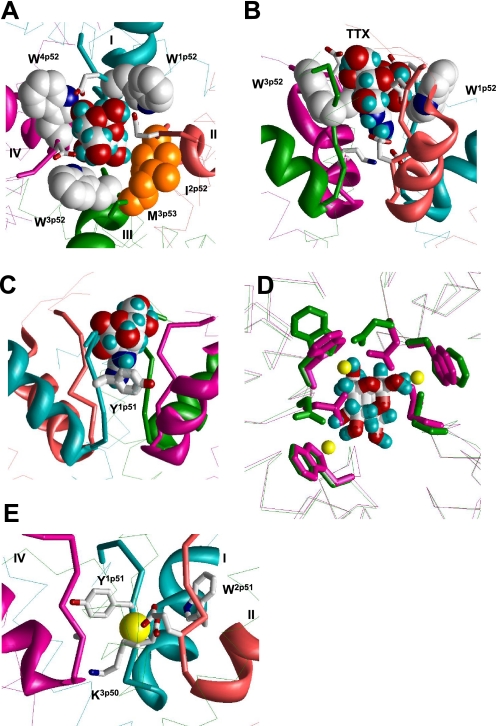
FIGURE 2.
Binding of TTX and Ca2+ in the revised model of NaV1.4. A and B, top and side views of TTX-bound channel. Backbones in repeats I, II, III, and IV are cyan, opaque, green, and magenta, respectively. P-helices are shown as ribbons, ascending limbs as thick α-tracings, and S6s as thin α-tracings. TTX fits perfectly in the outer pore and interacts with known TTX-sensing residues. C, cation-π interaction between Y1p51 and guanidine group of TTX (22). This interaction is achieved by only a modest deformation of our previous model (5), in which Y1p51 faces the pore and interacts with TTX by the aromatic ring edge rather than the aromatic plane. D, superposition of α tracings of the current model, which is MC-minimized either in the presence of Na+ ions (yellow Na+ ions, magenta side chains and backbones) or TTX (green side chains and backbones). Side chains of W1p52, W3p52, W4p52, and TTX-sensing outer carboxylates E1p53, E2p53, and D4p53 are shown as sticks. The root mean square deviation between α carbons in these models is as small as 0.2 Å. Note that hydroxyl groups of the TTX molecule are very close to positions of Na+ ions. E, possible binding of a Ca2+ ion in the Na+ channel. The ion binds to acidic residues D1p50 and E2p50 of the DEKA locus and displaces the ammonium group of K3p50 toward the inner pore. The aromatic side chains of Y1p51 and W2p51 are close enough to the Ca2+ ion to interact with it through water molecules in the first hydration shell. The complex likely corresponds to the blocked channel state. In the absence of another cation-binding site in the narrowest part of the outer pore, an incoming ion cannot displace the bound Ca2+ ion.