FIGURE 5.
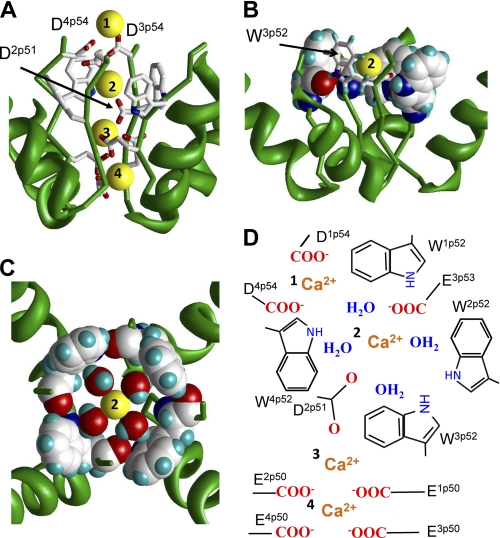
Possible role of exceptionally conserved tryptophans in positions p52 of calcium channels. A, side view of the predicted pattern of Ca2+ binding in the CaV1.2 model. The most extracellular Ca2+ ion is coordinated by the outer carboxylates D1p54 and D4p54. The outer carboxylate E3p53 and highly conserved D2p51 coordinate the hydrated Ca2+ ion in the Wp52 ring. Two Ca2+ ions at lower levels of the outer pore are coordinated by the selectivity filter glutamates. B, side view at a Ca2+ ion in the ring of W52s. Residues W1p52, W2p52, and W4p52 are space-filled, and W3p52 is shown by sticks. C, top view at ring of four Wp52s, which is stabilized by inter-repeat H-bonds. Each H-bond involves the side chain NH group in the given repeat and the backbone carbonyl in the next repeat. In this arrangement, the tryptophan side chains form a cation-attractive path by maximizing exposure of π-electrons to the pore lumen. The size of the tryptophan-ring inner surface matches the dimension of the Ca2+ ion with the first hydration shell. Four water molecules and the Ca2+ ion lie in a plane, which is normal to the pore axis. D3p54 and highly conserved D2p51 provide two axial coordination bonds (not shown) to complete the hexa-coordinated geometry, which is seen in x-ray structures and predicted by molecular dynamics simulations (see text). The water oxygens interact with the Ca2+ ion, while water hydrogens interact with π-electrons of the tryptophans. D, schematic view of model A. Calcium ions are labeled with numbers.