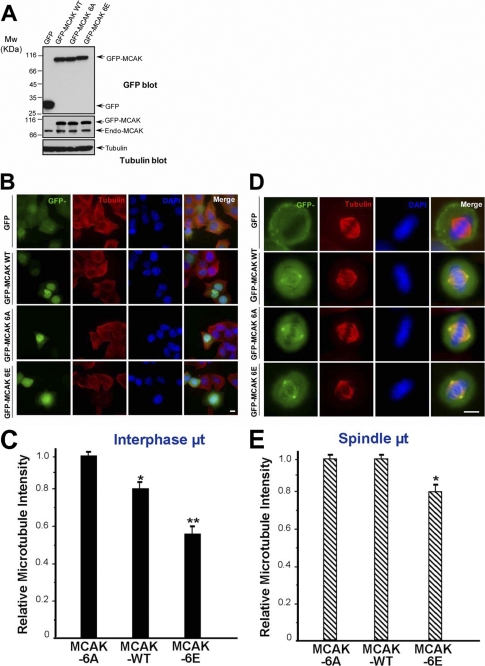
FIGURE 4.
PLK1 phosphorylation stimulates the MT depolymerase activity of MCAK. A, expression levels of GFP-MCAK and its phosphorylation site mutants in HeLa cells. HeLa cells were transfected with GFP, GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E. At 36 h post-transfection, cells were harvested, boiled in the SDS-PAGE buffer, and analyzed by Western blotting with an anti-GFP antibody (top panel), anti-MCAK antibody (middle panel), or anti-tubulin antibody (as a loading control, bottom panel). B, in vivo analysis of GFP-MCAK and its phosphorylation site mutants in interphase cells. HeLa cells were transfected with GFP, GFP-MCAK WT, GFP-MCAK 6, or GFP-MCAK 6E. At 36 h post-transfection, cells were fixed with methanol and stained for α-tubulin (red) and DAPI (for DNA, blue), respectively. C, statistical analysis of the relative MT density in B as described under “Materials and Methods.” Data are presented as means ± S.E. and derived from 75 cells from three independent experiments. *, p < 0.05; **, p < 0.01. D, in vivo analysis of GFP-MCAK and its phosphorylation sites mutants in mitotic cells. HeLa cells were transfected with GFP, GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E and synchronized to metaphase. At 36 h post-transfection, cells were fixed and stained as described in B. E, statistical analysis of relative spindle MT density in D as described under “Materials and Methods.” Data are presented as means ± S.E. and derived from 33 cells from three independent experiments. *, p < 0.05.