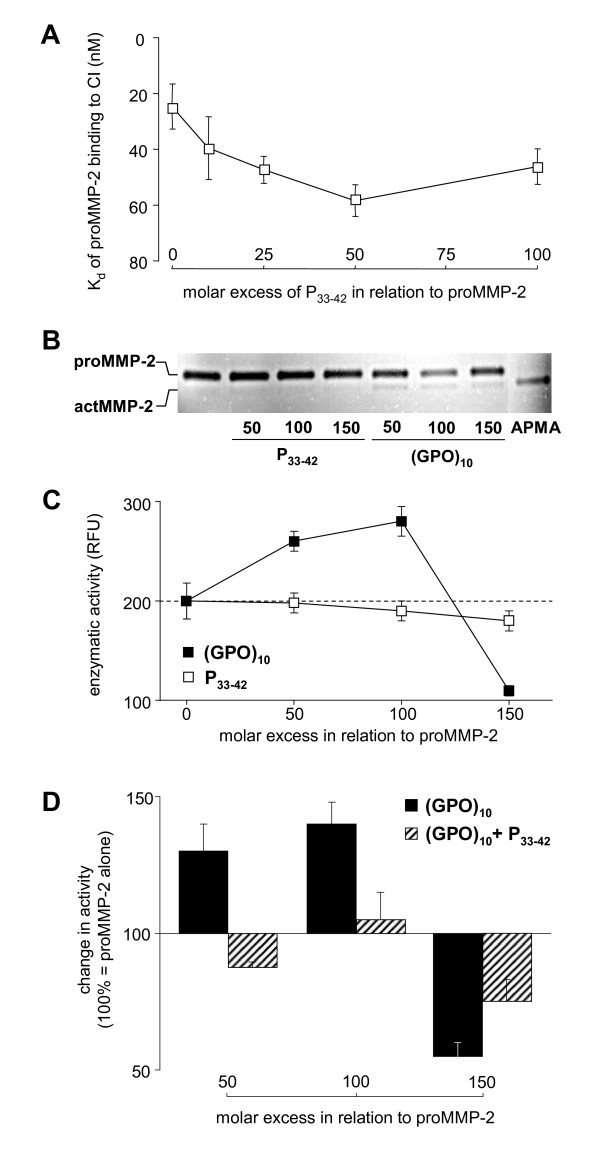
Figure 6.
Comparison of the effects of MMP-2 prodomain-derived collagen-binding domain (CBD)-binding peptide (P33-42) and (GPO)10 on proMMP-2 binding and activation. (A) Up to 100-fold molar excesses of P33-42 were mixed with 100-250 nM proMMP-2 in MMP activity buffer. Enzymatic activity was specifically blocked by 1 μM Ro 28-2653 before passing over CI immobilized to a sensor chip. Kd values were calculated from the sensorgrams for each P33-42 concentration and are shown as mean values ± SD. (B) Mixtures of 50 ng of proMMP-2 with P33-42 or (GPO)10 were subjected to substrate gel zymography after 2-h incubation at room temperature. ProMMP-2 (72 kDa) or actMMP-2 (62 kDa) after treatment of proMMP-2 with aminophenyl mercuric acetate served as controls for the activation state. Shown is one representative of three independent experiments. (C) Dye quenched (DQ)-gelatine was incubated with 10 ng of proMMP-2 in MMP activity buffer in the presence of up to 150-fold molar excesses of (GPO)10 or P33-42. After 5 h at 25°C, end point fluorescence was determined. Results are mean values ± SD of three independent experiments. (D) ProMMP-2 was added to up to 150-fold molar excesses of (GPO)10 alone or to equimolar mixtures of P33-42 and (GPO)10 before incubation with DQ-gelatine. Substrate cleavage as measured after 5 h at 25°C was calculated in relation to proMMP-2 alone (100%). Results are mean values ± SD of three independent experiments.