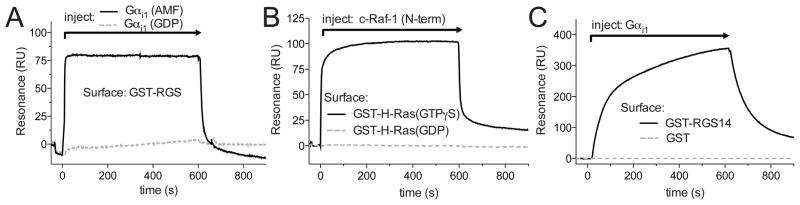
Figure 3. Diverse applications of an anti-GST CM5 sensor chip.
A. A GST-RGS fusion protein was immobilized on the surface of flow cell 1 and GST was immobilized on the surface of flow cell 2 (the latter surface to use to subtract sensorgram changes in resonance due to buffer shifts). Gαi1 pre-incubated with either GDP or GDP+Mg2++AlF4− (AMF), a transition-state mimetic form known to bind avidly to RGS proteins, was injected over the sensor surface. GST-RGS bound to the Gαi1(AMF) but not Gαi1(GDP) as expected. B. GST-H-Ras loaded with a non-hydrolyzable GTP analogue, GTPγS, was loaded in flow cell 1, GST-H-Ras loaded with GDP was loaded in flow cell 2, and GST control was loaded in flow cell 3. An N-terminal construct of c-Raf-1, a known H-Ras binding partner, was injected across the sensor chip surface. The data show the nucleotide state selectivity of the H-Ras/c-Raf-1 interaction. C. GST-RGS14 was loaded in flow cell 1 and GST was loaded in flow cell 2. Gαi1 was injected in GDP+Mg2++AlF4− (AMF) Running Buffer over the sensor chip surface and a Gαi1/RGS14 specific interaction was detected. Specific binding for all injections was determined by subtracting the non-specific binding observed from a GST control flow cell from the experiment-containing flow cells.