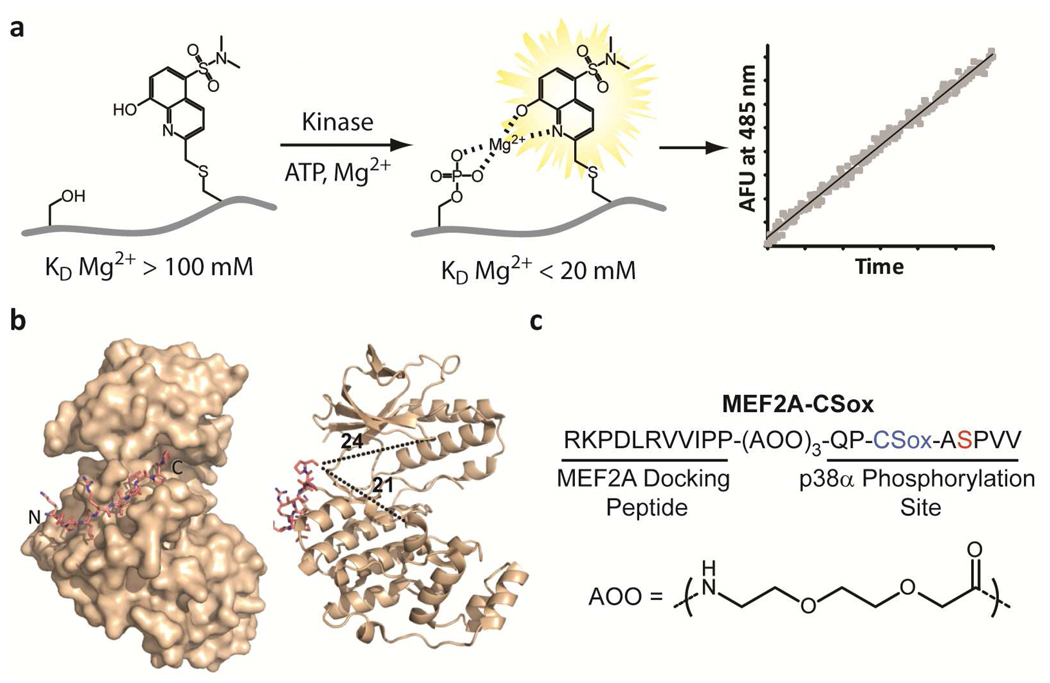
Figure 1.
Rational design of a p38α chemosensor. a) A schematic of the chelation-enhanced fluorescence of the CSox amino acid upon phosphorylation. b) A crystal structure of the MEF2A docking peptide bound to p38α (left) and distances in Å from the C-terminus of the docking peptide to representative distal regions of the catalytic and substrate-binding domains of the kinase are shown (right) (18). c) The amino acid sequence of the MEF2A-CSox sensor with the position of CSox (blue) and the site of phosphorylation (red) indicated. The flexible 8-amino-3,6-dioxaoctanoic acid (AOO) linker is also shown.