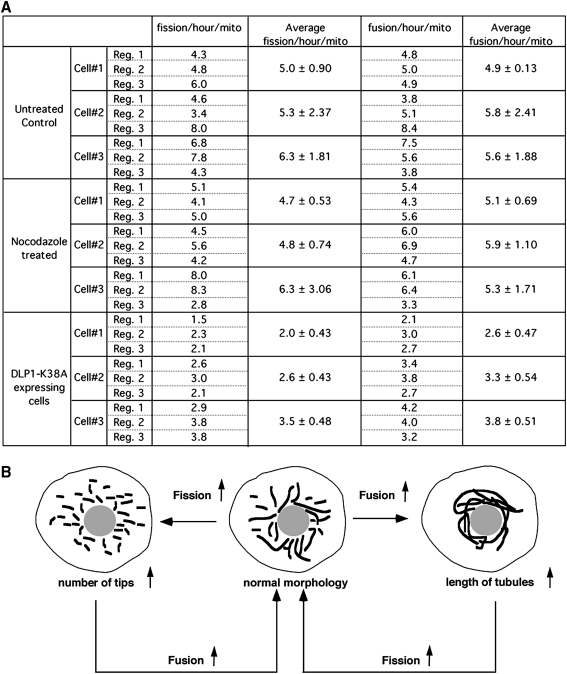
FIG. 2.
Control of fission/fusion balance. (A) Quantification of fission and fusion frequencies from time-lapse sequences acquired in every 10 s for 100 frames. Time-lapse images from normal, nocodazole-treated, and dynamin-like protein 1 (DLP1)-K38A mutant cells were subjected to the quantification. Fission events were counted when a single mitochondrion became two by a spatial separation that sustained at least two consecutive frames. Similarly, fusion was counted when separate mitochondria became converged and the connection sustained at least for two consecutive frames. Total number of fission and fusion were divided by the number of mitochondria that were present at the initial frame of the time-lapse sequence, and the frequency of fission and fusion was expressed as the events/hour/mitochondrion. Three regions were selected within a cell and the average fission and fusion were calculated. The quantification was repeated in a total of three cells for each treatment. The frequency of fission is similar to that of fusion within each region (∼5–6 events/hour/mito), demonstrating a balance between fission and fusion. Nocodazole treatment did not change the fission and fusion frequency, indicating that microtubules are not essential for these processes. Expression of DLP1-K38A resulted in a twofold decrease in both fission and fusion. (B) A proposed feedback mechanism to maintain a balance between mitochondrial fission and fusion. Frequency of mitochondrial fusion is predicted to be proportional to the number of mitochondrial tips. Likewise, the number of fission events is likely to increase as tubule length increases. In cells with reduced fission (e.g., DLP1-K38A expressing cells), fusion frequency also decreased due to the decrease of the number of mitochondrial tips available for fusion (A). In healthy cells with properly working fission and fusion machineries, the elongation of mitochondrial tubules due to increased fusion would increase fission frequency, which rapidly restores normal mitochondrial shape.